Chemicals in Medicine: Class 12
Chemicals
play a crucial role in medicine, whether as active pharmaceutical ingredients
(APIs), excipients, or components of medical devices. Here are some key
chemicals commonly used in medicine:
Acetylsalicylic
Acid (Aspirin):
Used as an analgesic (pain reliever), antipyretic (fever reducer), and
anti-inflammatory agent.
Acetaminophen
(Paracetamol): Another
common analgesic and antipyretic used to relieve pain and reduce fever.
Ibuprofen: Nonsteroidal anti-inflammatory
drug (NSAID) used for pain relief and reducing inflammation.
Penicillin: A group of antibiotics derived from
Penicillium fungi, used to treat bacterial infections.
Insulin: Hormone used to treat diabetes
by regulating blood sugar levels.
Epinephrine
(Adrenaline):
Used in emergency situations (e.g., anaphylaxis) to treat severe allergic
reactions and asthma attacks.
Morphine: Opioid analgesic used to relieve
severe pain, particularly post-surgical or in cancer patients.
Corticosteroids: Synthetic drugs similar to
corticosteroid hormones produced by the adrenal glands, used to treat
inflammatory conditions such as asthma, arthritis, and allergic reactions.
Chemotherapy
Drugs: Various
chemicals used to treat cancer by inhibiting or killing cancer cells, including
methotrexate, cisplatin, and doxorubicin.
Antidepressants: Various classes of chemicals
used to treat depression and other mood disorders, including selective
serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake
inhibitors (SNRIs), and tricyclic antidepressants (TCAs).
Antihistamines:
Drugs used to
treat allergies and allergic reactions by blocking the action of histamine,
including diphenhydramine, loratadine, and cetirizine.
Anesthetics: Various chemicals used to induce
anesthesia and numb sensation during medical procedures, including lidocaine,
propofol, and isoflurane.
Vaccines:
Various
chemicals are used in the production and stabilization of vaccines, including
adjuvants (e.g., aluminum salts), preservatives (e.g., thiomersal), and
stabilizers.
Antacids
and Antireflux Agents:
Chemicals used to treat gastrointestinal conditions such as acid reflux and
peptic ulcers, including proton pump inhibitors (PPIs) and H2-receptor
antagonists.
Anticoagulants: Chemicals used to prevent blood
clotting, including heparin, warfarin, and direct oral anticoagulants (DOACs).
These
are just a few examples of the wide range of chemicals used in modern medicine
to diagnose, treat, and manage various health conditions. Each chemical has
specific properties and mechanisms of action that contribute to its therapeutic
effect.
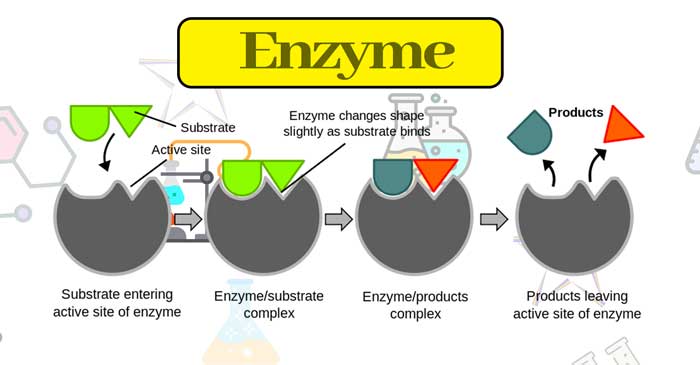
Chemicals in Medicine: Drugs compete with the natural substrate for their attachment to the active sites of enzymes. Such drugs are called competitive inhibitors. Some drugs do not bind to the enzyme’s active site. These bind to a different site of enzyme which is called allosteric site.
This binding of inhibitor at the allosteric sites changes the shape of the active site in such a way that the substrate cannot recognize it. If the bond formed between an enzyme and an inhibitor is a strong covalent bond and cannot be broken easily, then the enzyme is blocked permanently. The body then degrades the enzyme-inhibitor complex and synthesize the new enzyme.
Antibiotics are chemical substances (prepared wholly or partially by chemical synthesis) which in low concentration either kill or inhibit the growth of microorganisms by intervening in their metabolic processes. E.g.,
Dysidazirine – Toxic to cancer cells.
Drugs: These are the chemicals of low molecular masses (100-500 u) which interact with macromolecular targets and produce a biological response.
Medicines: These are the drugs that are therapeutic and used for diagnosis, prevention, and treatment of diseases.
Use of chemicals for therapeutic effect is called chemotherapy.
Classification of drugs: Drugs are classified on the basis of

Drug Target Interaction
Enzymes as a drug target: Drugs inhibit any of the two activities of the enzymes, they can block the binding site of the enzyme and prevent the binding of the substrate or they can inhibit the catalytic activity of the enzyme.
Receptors as a drug target: Proteins that transmit the communication to the different parts of the body are called receptors. Receptor proteins are embedded in the cell membrane and the receptor changes its shape to accommodate a chemical messenger which brings about transfer of message into the cell.
Read more Topics:
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
Drug Interact with Receptors in two ways:
– Drugs bind to their receptor sites and inhibit its natural function (antagonists). These are useful when blocking of message is required.
– Some drugs mimic the natural messenger by switching on the receptor (agonists). These are useful when there is a lack of natural chemical messenger.
The therapeutic action of different classes of drugs:
Antacids: These are chemicals that neutralize excess acid in the gastric juices and give relief from acid indigestion, acidity, heartburns, and gastric ulcers, e.g., magnesium hydroxide, calcium carbonate, etc.

Antihistamines: They diminish or abolish the main action of histamine released in the body and hence, prevent allergic reactions, they are also called anti–allergic drugs, e.g., diphenhydramine (Benadryl), pheniramine maleate (avil), etc.
Tranquilizers: These are chemical substances used for the treatment of stress, mild and severe mental diseases. They are neurologically active drugs and are also called psychotherapeutic drugs, e.g., veronal Amytal, Seconal, Equanil, chlordiazepoxide, etc. Veronal, Amytal, and seconal are called barbiturates. Barbiturates are hypnotic, i.e., sleep producing agents.
Analgesics
These are chemical substances that reduce pain. They are classified as:
— Non-narcotic analgesics: Aspirin and paracetamol belong to this class of drugs. They are effective in relieving skeletal pain such as that due to arthritis. They have many other effects such as reducing fever and preventing platelet coagulation. Aspirin finds use in the prevention of heart attacks because of its anti-blood clotting action.
— Narcotic analgesics: Morphine and many of its homologs, when administered in medicinal doses, relieve pain and produce sleep. In poisonous doses, they cause convulsions and ultimately death. These are mainly used for the relief of postoperative pain, cardiac pains and pains of terminal cancer.
- Mole in chemistry: What is it and how to calculate it?
- What are the common uses of cesium in industry : Properties, Isotopes
- What is aluminum bromide and how is it used in industry?
- Exploring the Rankine Scale: Understanding the Basics and Applications
- What is Empirical Formula? How to Calculate Empirical Formula?
These are chemical substances used to cure infections due to microorganisms, e.g., sulphadiazine, sulphadoxine, etc. Antibiotics, antiseptics and disinfectants are antimicrobial drugs.
– Antibiotics: These are chemical substances produced wholly or partly by chemical synthesis which in low concentration inhibit the growth or destroy microorganisms by intervening in their metabolic processes.
The antibiotics may be either bacteriocidal (kill the organisms in the body) e.g., penicillin, ofloxacin, etc., or bacteriostatic (inhibit the growth of organisms), e.g., erythromycin, chloramphenicol, etc.
Antibiotics that kill or inhibit a wide range of Gram-positive and Gramnegative bacteria are said to be broad-spectrum antibiotics, e.g., tetracycline, chloromycetin and chloramphenicol.

Those effective mainly against Grampositive or Gram-negative bacteria are narrow-spectrum antibiotics, e.g., penicillin-G.
— Antiseptics: These are chemicals which either kill or prevent the growth of microorganisms and are applied to the living tissues such as wounds, cuts, ulcers and diseased skin surfaces.
Dettol is a commonly used antiseptic and it is a mixture of chloroxylenol and terpineol
Bithionol is added to soaps.
Tincture of iodine, i.e., 2-3% solution in the alcohol-water mixture is applied on wounds.
– Disinfectants: These are also used to kill microorganisms, but they are applied to inanimate objects.
Some substances can act as antiseptic as well as a disinfectant by varying the concentration.
0.2% phenol is an antiseptic, whereas its 1% solution is disinfectant.
0.2-0.4 ppm chlorine in aqueous solution acts as a disinfectant.
- Mole in chemistry: What is it and how to calculate it?
- What are the common uses of cesium in industry : Properties, Isotopes
- What is aluminum bromide and how is it used in industry?
- Exploring the Rankine Scale: Understanding the Basics and Applications
- What is Empirical Formula? How to Calculate Empirical Formula?
Antifertility drugs: Chemical substances used to prevent conception or fertilization are called antifertility drugs. These are essentially a mixture or estrogen and progesterone derivatives which are more potent than the natural hormones, e.g., mifepristone, ormeloxifene, etc.
Chemicals in Food
Preservatives: These are the chemical substances that are added to the food materials to prevent their spoilage and to retain their nutritive value for long periods. These preservatives prevent the rancidity of food and inhibit the growth or kill the microorganisms.
The preservation of food by adding a sufficient amount of salt to it is called salting. Salt prevents the water from being available for microbial growth.
The microbial growth in food materials can also be prevented by adding certain chemical substances. The most common preservative used is sodium benzoate (C6H5COONa). It is metabolized by conversion to hippuric acid, C6H5CONHCH2COOH which ultimately is excreted through urine.
Certain food preservatives such as BHA and BHT are used for edible oils, also act as antioxidants.
Artificial sweetening agents: These are chemical compounds which give sweetening effect to the food and enhance its odour and flavor.
Antioxidants: These are the chemical substances that prevent oxidation and subsequent spoilage of the food. These act as sacrificial materials, i.e., they are more reactive towards oxygen than the materials they are protecting. They also reduce the rate of involvement of free radicals in the ageing process.
Read more Topics:
- Halogenation Reaction|| What is the Mechanism of Halogenation?
- Chemical Properties of Alkanes || What are Examples of Alkanes?
- Atomic Structure Chemistry || How do you find the Atomic Structure?
- Periodic Table Elements|| How many Elements are in the Periodic Table?
- Exciting and Entertaining Chemical Tricks || List of Nobel Prize in Chemistry
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
Soaps
These are sodium or potassium salts of long-chain fatty acids, e.g., stearic, oleic and palmitic acids. Soaps containing sodium salts are prepared by heating glyceryl ester of fatty acid with aqueous NaOH solution and the reaction is known as saponification.
Only sodium or potassium soaps are soluble in water and are used for cleaning purposes. Generally, potassium soaps are soft to the skin than sodium soaps.
Detergents
These are the materials that are used for cleaning purposes. They are also called soapless soaps.
Anionic detergents: Their polar head is negatively charged.
Cationic detergents: Their polar head is positively charged e.g.
These are used as a fabric softener and hair conditioner.
Non-ionic detergents: Their polar head is neutral. e.g.,
The action of soap in hard water: Hard water contains Ca2+ or Mg2+ ions which react with sodium or potassium salt of fatty acid (soap) to form calcium or magnesium salt of fatty acids called scum.
Advantages of synthetic detergents over soaps:
They can be used in hard water, in acidic medium while soaps get precipitated.
They are more soluble in water and thus, form lather more easily.
They are stronger cleansing agents than soaps as they decrease the surface tension to a greater extent.
