Uranium Uses || What is Uranium Used for || How is Uranium Formed
Uranium is an element with an atomic number of 92, and its element symbol is U, which is the heaviest element that can be found in nature.
There are three isotopes in nature, all of which are radioactive and have very long half-lives (hundreds of thousands to 4.5 billion years).
In addition, there are 12 artificial isotopes (226U ~ 240U). Uranium was discovered in 1789 by Martin Heinrich Klaproth. Uranium compounds were used to color porcelain early and were used as nuclear fuel after nuclear fission was discovered.
Introduction
The heaviest metal produced naturally. It is silver-white, with strong hardness, high density, ductility, and radioactivity. Uranium is generally found in the combination of uranium with oxygen, oxides or silicates. Uranium atom can undergo fission reaction and release a large amount of energy so that it can be used in power generation, nuclear weapon manufacturing and other fields.
The Second World Coalition’s nuclear weapons program triggered demand for uranium, and uranium production came into being. By the 1970s, the uranium production industry had been firmly established.
Chemical properties
Uranium is a Group III b actinide radioactive chemical element, symbol U, atomic number 92, relative atomic mass 238.03, natural element with the largest atomic number and relative atomic mass. Uranium is a dense silver-white metal at room temperature the new section of uranium was shiny steel gray, but a black oxide film gradually formed in room temperature air.
The outer electron layer configuration of the uranium atom is [Rn] 5f 3 6d 1 7s 2, and the shell of 5f 3 6d 1 7s 2 is a valence electron. Uranium has four valence states of +3, +4, +5, and +6, and the main valence states are +4 and +6.
Uranium is a very positively active element that reacts with almost all non-metallic elements (except inert gases) to form compounds, often in the form of U 3+, U 4+, UO2 + and UO 2 2+ ions. Uranium and hydrogen react reversibly at 523K to form UH3. The uranium-oxygen system is relatively complicated.
There are multiple phases between UO2 and UO3. The Important oxides are UO2, U3 O8 and UO3. Among them, UO 2 is currently the most widely used nuclear fuel. Uranium and halogens are important compounds in the preparation of nuclear fuel.
For example, UF4 is an intermediate product for the production of metallic uranium and UF6. The triple point of UF6 is 337K, which is the raw material for gaseous uranium isotope separation. Uranium carbide, uranium nitride, and uranium silicide are all considered promising nuclear fuels with superior performance.
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
Metal uranium darkens in the air and can be corroded by steam and acids, but resistant to alkali corrosion. Its atomic radius is 138.5pm; the ionic radii of U 3+ , U 4+ , U 5+ , and U 6+ are 103, 97, 89, and 80 pm, respectively. The electronegativity of uranium was determined by Pauling to be 1.38; Allred and Rochow were determined to be 1.22.
Uranium can react with most non-metallic elements and their compounds. The reaction temperature and reaction speed vary with the particle size of uranium.
Uranium can spontaneously ignite in air or oxygen at room temperature, and fine uranium can spontaneously ignite in water. Under certain conditions, the energy released by the oxidation of uranium can cause an explosion. The lower limit of the explosive concentration of uranium dust is 55 mg / dm3. Uranium can react with many metals to form intermetallic compounds. Uranium can form solid solutions with niobium, hafnium, zirconium, molybdenum, and titanium.
Uranium and its compounds are highly chemically toxic. The allowable concentration of soluble uranium compounds in the air is 0.05 mg / m3, the allowable concentration of insoluble uranium compounds is 0.25 mg / m 3, and the allowable human radioactive dose to natural uranium is soluble. The uranium compound is 7400Bq and the insoluble uranium compound is 333Bq.
Physical properties
Uranium is a radioactive metal element that can be used as a fuel for nuclear reactions. Uranium is a silver-white metal, almost as hard as steel, with a high density (relative density of about 18.95), a melting point of 1135°C and a boiling point of 4134°C. Before the development of nuclear energy, it was used to make yellow glass.
Uranium is the element with the highest atomic number in nature. E. Paley (1811-1890) isolated metallic uranium in 1841, although uranium was previously recognized in pitch uranium mines. It is also hidden in mica uranium ore, vanadium potassium uranium ore, and monazite.
There are three allotropes of uranium, their temperature and main structural characteristics are listed in the table. The density of α-U at room temperature was 19.02 t/m3. α-U and β-U have obvious anisotropy.
For example, between 298 and 523 K, the thermal expansion coefficients of α-U single crystals along the a, b, and c axes are α a = + 33.24 × 10-6 / K, Α b = -6.49 × 10-6 / K, α c = + 30.36 × 10 -6 / K. γ-U has an isotropic structure.
The thermal expansion coefficient of the randomly arranged polycrystalline uranium in the range of 293 to 373 K is equal to 16.3 × 10 -6 / K.
The specific heat between 5 and 350K is 27.66J / (mol · K). The thermal conductivity of α-U increases with increasing temperature. It is 25.1W / (m · K) at room temperature and 37.7W / (m · K) at 1033K.
The mechanical properties of uranium vary with the sample furnace number and heat treatment. For α-rolled and annealed samples, the maximum yield strength at room temperature is 206.8-275.8 MPa.
For small deformations of extruded uranium, the room temperature tensile strength limit is 586.1 ~ 861.8MPa.
Uranium has three lattice structures: α-U is an orthorhombic structure, a = 284.785pm, b = 585.801pm, c = 494.553pm; β-U is a square structure, a = 1076.0pm, c = 565.2pm, For body-centered cubic structure, a = 352.4pm. Their transition temperatures are 941K (α → β) and 1047K (β → γ).
Nuclear Properties
The thermal neutron absorption cross-section of uranium is 7.60b ± 0.07b. There are 15 isotopes of uranium (including isonuclear isotopes), with masses ranging from 227 to 240. The natural isotopic composition of uranium is listed in the following table.
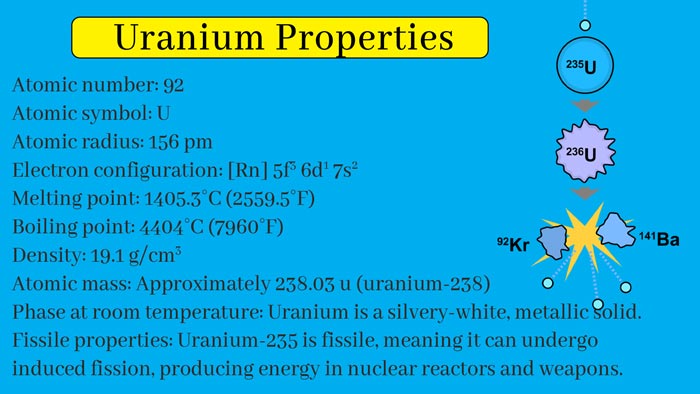
235U is the progenitor nuclide of the plutonium uranium decay system, 238U is the progenitor nuclide of the uranium and radium system, 234U is the decay product of 238U. 235U is the only natural fissile nuclide.
The 235U nuclides are bombarded by thermal neutrons and undergo fission (induced fission) after absorbing a neutron. A 235U nucleus emits total energy of 195 MeV during fission and simultaneously emits 2 to 3 (average 2.5) neutrons.
As long as one of the neutrons causes fission of the other 235U nucleus, the chain nuclear fission will continue. 238U is not a fission nuclide, but 238U generates 239U after neutron absorption in the active area of the reactor, and 239 U undergoes two beta decays to generate fissionable Pu.
Therefore, the fast neutron breeder reactor can be used to fully play the role of 238U and improve the utilization of natural uranium.
Isotope and its Half-life
Natural uranium contains three isotopes: 238U, 235U, and 234U. Their contents are 99.28% (238U), 0.71% (235U), and 0.006% (234U), and their half-lives are (238U) and 4.51. × 109, (235U) 7.09 × 108 and (234U) 2.35 × 105 years. Among them, 235U is the most important, which is currently the fuel of nuclear power.
A 235U nucleus emits about 2.5 neutrons when it absorbs a thermal neutron, and releases 207 MeV energy. The energy released by 1 kg of 235U nuclear fission is equivalent to the energy produced by burning 2700t coal.
Depending on the reactor type and its operating conditions, nuclear fuel can be natural uranium or enriched uranium with increased U content.

The separation of uranium isotopes by gas diffusion method, centrifugation method or laser method can make the enrichment of U more than 90%. U captures neutrons and transforms them into fissile Pu. Pu and U are also the main raw materials for nuclear weapons.
In 25km crust contains 10 14 T U, wherein water containing 10 10 T, average water per ton of uranium 3.3mg. Hundreds of uranium-containing minerals exist in nature, but most of them are depleted, so economically large-scale mining is difficult.
At present, uranium ore with economic value is about 0.1% of U 3 O 8 content. If fast neutron breeder reactors are developed, the utilization of uranium resources can be increased by 60 to 70 times compared to pressurized water reactors.
The most abundant uranium isotope is 238U, and the most abundant is 235U, which can be used as fuel for nuclear power generation. The least abundant is 234U. There are also 12 artificial isotopes.
| isotope | Abundance | half-life | Decay mode | Decay Energy (MeV) | Decay products |
| 232U | Man-made | 68.9 years | Spontaneous division | – | – |
| alpha decay | 5.414 | Th-228 | |||
| 233U | Man-made | 159200 | Spontaneous division | 197.93 | – |
| alpha decay | 4.909 | Th-229 | |||
| 234U | 0.006% | 245500 | Spontaneous division | 197.78 | – |
| alpha decay | 4.859 | Th-230 | |||
| 235U | 0.72% | 7.038 ×10^8 years | Spontaneous division | 202.48 | – |
| alpha decay | 4.679 | Th-231 | |||
| 236U | Man-made | 2.342× 10^7 years | Spontaneous division | 201.82 | – |
| alpha decay | 4.572 | Th-232 | |||
| 237U | Man-made | 6.75 days | beta decay | 0.519 | Np-237 |
| 238U | 99.275% | 4.468× 10^9 years | Spontaneous division | 205.87 | – |
| alpha decay | 4.270 | Th-234 |
Uranium Alloy
Uranium can form intermetallic compounds with many metals. Uranium has such defects as lively chemical properties, poor anisotropic structure and poor mechanical properties.
Certain properties of uranium alloys are superior to metallic uranium, which is important in the manufacture of nuclear fuel elements.
Adding appropriate amount of other metals, such as niobium, chromium, molybdenum or zirconium, can improve the thermal conductivity, crystal structure and metallographic structure, heat treatment characteristics, radiation stability and corrosion resistance of uranium.
Depleted uranium bomb is a kind of high-efficiency combustion armor-piercing bomb made of depleted uranium alloy. It is made by virtue of the density of depleted uranium material (2 times of lead density), high strength (3 times of steel strength), strong penetrating power, and easy burning, Depleted uranium alloy contains 238U, 235U, etc. The residual 235U after the depleted uranium bomb can damage the kidneys and nervous system and cause lung cancer. Depleted uranium alloys with high density and hardness can also be made into radiation-proof materials.
Uses of Uranium
Prior to 1942, uranium was mainly used as a colorant for glass and ceramics, and was used in small amounts. With the discovery of the 235U chain nuclear fission reaction, the huge energy released by nuclear fission (the fission energy released by 1 kg of 235U is equivalent to 1800tTNT explosives) has attracted people’s attention. It was first used to make atomic bombs and hydrogen bombs.
Uranium nuclear reactor
Since the late 1950s, uranium has been increasingly used as a nuclear fuel for nuclear power generation. The energy released by 1kg 235U nuclear complete fission is equivalent to the energy released by burning 2700t high-quality coal.
In addition, uranium nuclear reactors can also be used as radiation sources for agricultural irradiation breeding, food industry food preservation and sterilization, and for the production of man-made elements.

In medicine, it is used for radiotherapy, radio immunity kits, diagnostic imaging, etc. In industry and geology, it is used for industrial flaw detection, automatic control, geological exploration and cultural archaeology, etc.
Scientific research and industrial practice prove that uranium is the only natural nuclear fuel, and the nuclear energy industry must rely on uranium.
Because the nuclear energy industry has both peaceful and military applications, uranium has become a special commodity metal, and its production is affected by many political, social and economic factors.
In the 1940s and 1950s, uranium was mainly used for nuclear weapons, and after the 1950s it was mainly used for nuclear power generation.
The world’s uranium production has been oversupply for a long time, and there are large stocks. The price of U 3 O 8 per kilogram in the international market decreased from US $ 97 in early 1978 to US $ 19.84 in 1990.
The annual output of uranium in western countries also decreased from 43960t in 1980 to 35278t in 1985. But during this period, nuclear power plants developed rapidly, with a total installed capacity of 135 million kW in 1980 and an increase to 318 million kW in 1989.
The annual production of uranium in 1985 was lower than the demand for nuclear power.
Atomic bomb
Regularly place conventional explosives around uranium, and then use electronic detonators to make these explosives accurate
At the same time, the huge pressure generated by the explosion pressed the uranium together and was compressed to reach the critical condition and an explosion occurred.
Or if two pieces of uranium whose total mass exceeds the critical mass are brought together, a violent explosion will occur. Critical mass refers to the mass of fissile material needed to maintain a nuclear chain reaction.
Different fissionable materials have different thresholds due to the nature of the nucleus (such as fission cross-section), physical properties, material shape, purity, whether it is surrounded by neutron reflecting materials, whether there are neutron-absorbing materials, etc. quality.
A combination that just happens to produce a chain reaction is called the critical point has been reached. With more mass combinations like this, the rate of nuclear reactions increases exponentially , called supercritical.
If the combination can carry out a chain reaction without delaying the release of neutrons, this criticality is called an immediate criticality and is a supercritical one. A critical combination will cause a nuclear explosion. If the combination is smaller than the critical point, fission will decrease over time, which is called subcritical.
Nuclear weapons must remain subcritical before they detonate. Taking the uranium nuclear bomb as an example, uranium can be divided into several large pieces, and the mass of each piece is maintained below the threshold. Quickly combine uranium blocks during detonation.
The “little boy” atomic bomb that was dropped on Hiroshima shot a small piece of uranium through a barrel and fired on another large piece of uranium, causing sufficient mass. This design is called a “gun style”.
Uranium nuclear fission
In nature, 234U does not undergo nuclear fission. Generally, 238U does not undergo fission. The only 235U is prone to nuclear fission. Nuclear fuel mainly refers to 235U. The half-life of 235U is 7.038 × 10 8 years. Starting from 235U, after 11 consecutive decays, a stable 207 Pb finally appears.
The half-life of 238U is 4.468 × 10 9 years. Starting from 238U, after 14 consecutive decays, the stable 206 Pb-206 finally appears. In the continuous decay of 238U, the longest half-life of the nucleus is 234U, and its half-life is 2.45 × 10 5 years.
235U, 233U, and 239 Pu are the main nuclear fissionable materials, which can be directly used as nuclear fuel. They can be obtained in large quantities and easily absorb slow neutrons (energy less than 1eV) and fission.
234U and 238U are not. 235U exists in natural uranium, and 233U and 239Pu are produced by uranium nuclear reactors. For 235U, 233U, and 239Pu, neutrons of any energy can split them and release energy; for 235U, the slower the neutron, the more likely it is to cause fission. 238U absorbs a neutron and can also be transformed into a fissionable matter.
Both 235U and 238U can spontaneously fission, but the probability of spontaneous fission is very small.
235U fission
Studies have shown that after 235U absorbs slow neutrons, there are more than 40 fission modes, which can produce at least 36 kinds of elements of more than 300 nuclides and fast neutrons (average 2.5), and release huge energy.
In addition to neutrons, uranium nuclear fission products usually have two (two-split) fission products, and three (three-split) and four fission products (founded by French physicist Qian Sanqiang and others in France in 1946). “Triple splits” are extremely unlikely. In addition to neutrons, there are many ways to combine the “two splits” of uranium nuclei.
The ratio of the masses of fragments is roughly 3: 2, and the chance of the same mass is the smallest. It is an alpha particle, and the probability of occurrence of the “triple split” is 3/1000 of the “second split”.
Statistics show that the neutron energy (kinetic energy) emitted by 235U fission is in the range of 0.1-20 MeV, with an average of 2 MeV.
Fast neutrons alone cannot produce a continuous fission chain reaction of natural uranium, nor can slow neutrons cause fission of 238U. Continuous fission reactions cannot occur in 238U. 235U and 240 Pu, etc.
In addition to neutrons that can trigger their nuclear fission, charged particles or gamma rays with sufficient energy can also trigger fission. In addition, uranium also generates capture resonances for neutrons of about 25 eV, that is, capture without fission.
235U has small binding energy, a low nuclear fission barrier, and neutrons of any energy can make it fission, and it has a slow neutron (neutron rate of 2.2 × 10 3 m / s, which is comparable to the gas molecule movement rate at room temperature).
In this way, it stays relatively near the uranium nucleus for a long time, and it is easy to hit the uranium nucleus and cause it to fission. 235U absorbs a slow neutron.
Usually, the excited state of 236U (re-nucleation) is formed, then it is split into two pieces, and neutrons and energy are released at the same time. In a thermal neutron (a kind of slow neutron) reactor, the thermal neutron fission cross section of 235U is 200 times larger than the thermal neutron fission cross section of 238U.
There will be a sufficient number of neutrons to cause 235U nuclear fission. This can make up for the weakness of less 235U content in natural uranium or enriched uranium, the utilization rate of uranium is 1% -2% when this reactor is working.
238U fission
238U (240Pu, 232Th) fission is valved, and neutrons less than 1.1 MeV will be absorbed or scattered by them, and cannot cause fission; neutrons with larger energy can make them fission, but the possibility is very small. 238U has large binding energy and a high fission barrier.
Fast neutrons with energy exceeding 1.4 MeV can make it fission and release a large amount of neutron energy. Studies have shown that 238U has many resonance absorption peaks above a few MeV, and its fission probability increases with increasing neutron energy.
238U is not prone to fission, but it can become better nuclear fission materials such as 239 Pu and 233U after neutron absorption. The probability of thermal neutrons being captured by 238U is about 1/190 of the probability that thermal neutrons will cause fission of 235U.
The main role of fast neutrons and 238U nuclei is inelastic collisions. Most neutrons reduce energy through inelastic collisions and are absorbed by 238U nuclei in multiple collisions.