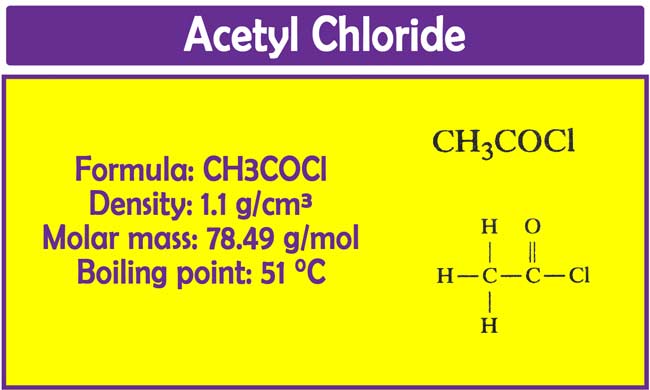
Acetyl chloride: How do you make Acetyl Chloride? | Properties | Uses
Laboratory Method
Preparation of Acetyl chloride
By the Reaction of acetic acid and PCl5 or PCl3: In the laboratory acetyl chloride is made by reaction of phosphorus tri chloride or penta chloride on Glacial acetic acid.
3CH3COOH + PCl3 → 3CH3COCl + H3PO3
CH3COOH + PCl5 → CH3COCl + POCl3 + HCl
A distillation flask of 500 ml capacity contains approximately 40 ml of glacial acetic acid. With the help of a cork with hole in the flask, a dropping funnel is attached and the distillation flask attaches to the condenser and receiver.
The receiver has a lateral tube filled with anhydrous calcium chloride that absorbs the hydrogen chloride produced in the reaction and does not allow the water vapor of the outside air to reach inside.

The point funnel fills approximately 25 ml of phosphorus tri chloride. The distillation flask is heated to about 45°C by placing it on a water heater. By doing this, all the hydrogen chloride gas formed in the reaction is removed. After that heat the flask to about 55°C.
At this temperature, acetyl chloride is distilled and collected in the receptor. Re-distillation gives pure acetyl chloride.
The point funnel fills approximately 25 ml of phosphorus tri chloride. The distillation flask is heated to about 45°C by placing it on a water heater. By doing this, all the hydrogen chloride gas formed in the reaction is removed. After that heat the flask to about 55°C.
At this temperature, acetyl chloride is distilled and collected in the receptor. Re-distillation gives pure acetyl chloride.
By the Reaction of acetic acid and Thionyl chloride: Acetyl chloride is also obtained by the action of acetic acid and thionyl chloride.
CH3COOH + SOCl2 → CH3COCl + SO2 + HCl
Industrial Production:
By the action of calcium acetate and sulfuryl chloride: acetyl chloride is obtained by distillation of anhydrous calcium acetate with sulfuryl chloride.
(CH3COO)2Ca + SO2Cl2 → 2CH3COCl + CaSO4
Physical Properties
It is a colorless and pungent liquid. Its boiling point is 52°C and relative density is 1.104. It decomposes quickly by water, so it absorbs the water vapor of the air and decomposes to form hydrogen chloride.
For this reason, leaving it open in the air, the smoke of hydrogen chloride gas comes out. It is soluble in ether, acetone and acetic acid.

Acetyl Chloride Chemical Properties
Water decomposition: It decomposes quickly by water and forms acetic acid and hydrogen chloride.
CH3COCl + HOH → CH3COOH + HCl
Acetylation: When oxygen in a compound is displaced by the hydrogen atom acetyl group(-COCH3) attached to nitrogen or sulphur atoms, this reaction is called acetylation. Acetylation is mainly done by reaction with acetyl chloride.
Example:
Reaction with Alcohol: It makes ester by reacting with alcohol.
CH3COOCl + HOC2H5 → CH3COOC2H5 + HCl
Reaction with Ammonia: It reacts with ammonia to produce acetamide.
CH3COCl + HNH2 → CH3CONH2 + HCl
Action from primary and secondary amines: It forms N –
substituted amide by acting with these amines.
CH3COCl + HNHC2H5 → CH3CONHC2H5 + HCl
CH3COCl + HN(C2H5)2 → CH3CON(C2H5)2 +HCl
Action from hydrazine hydroxyl amine and urea: It makes acetyl derivative by acting separately from them.
CH3COCl + HNHNH2 → CH3CONHOH2 + HCl
CH3COCl + HNHOH → CH3CONHOH + HCl
CH3COCl + HNHCONH2 → CH3CONHCONH2 + HCl
Reaction with anhydrous sodium acetate: It forms acetic anhydride when heated with anhydrous sodium acetate.
CH3COCl + CH3COONa → (CH3CO)2O + NaCl
Reduction: It is reduced by hydrogen in the presence of palladium containing barium sulphate to form acetaldehyde. This reaction is called Rosenmund Reduction.
CH3COCl + H2 → CH3CHO + HCl
It is reduced by lithium aluminium hydride (LiAlH4) to form ethanol.
CH3COCl → CH3CH2OH + HCl
The above reduction can also be done by H2 / Ni, Na / alcohol or NaBH4.
Friedel-Crafts reaction: It reacts with benzene in the presence of anhydrous aluminium chloride to form acetophenone.
C6H5H + ClCOCH3 → C6H5COCH3 + HCl
Grignard reagent: It reacts with Grignard reagents to form methyl ketone.
CH3COCl + ClMgCH3 → CH3COCH3 + MgCl2
Reaction with Ether: It reacts with di ethyl ether in the presence of anhydrous zinc chloride to form ethyl chloride and ethyl acetate.
CH3COCl + C2H5OC2H5 → C2H5Cl + CH3COOC2H5
Acetyl Chloride Uses
To find the number of -OH and -NH2 groups in carbonic compounds.
In the making of acetic anhydride, acetamide, acetaldehyde and other carbonic compounds
As acetylating reagent in the manufacture of dyes and medical
As reagent in the laboratory.