Aldehydes and Ketones: Preparation, Properties, Nomenclature
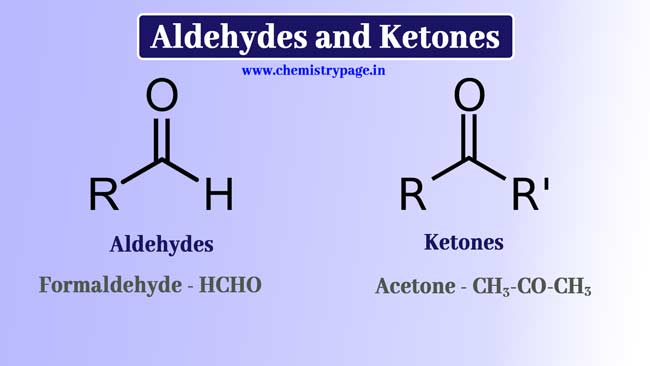
Aldehydes and Ketones
-CHO Group is called formyl group and -CO- Group is called a carbonyl group.
When the -CHO group is attached to a hydrogen or carbon atom, it is called an aldehyde group. And the related compound is called Aldehyde.
Example: -CHO group in HCHO is associated with H, so it contains aldehyde group and it is an aldehyde. In CH3CHO, -CHO is associated with group C, so it also has an aldehyde group and is also an aldehyde. -CHO group is present in HCOOH but it is associated with O, so aldehyde group is not present in this compound.
Compounds in which the -CHO group is attached to a hydrogen atom or an alkyl group are also called alkanal. The common formula of these compounds is CnH2nO.
The common formula of these compounds is RCHO where R is a hydrogen atom or an alkyl group. There is a difference of CH2 between the two consecutive alkanal.
Hence all alkanal are members of the same homogeneous category. The first member of these categories is formaldehyde (HCHO) and the second member is acetaldehyde (CH3CHO).
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
When the -CO-group is connected to carbon atoms on both sides, it is called keto group or Ketone group and the related compound is called Ketone.
Example: In CH3-CO-CH3 the -CO – group is connected to two methyl groups through carbon atoms, so a ketone group is present in it and it is a ketone. In CH3COOH, the -CO-group is present but on one hand it is connected to the -OH group with oxygen atom, so ketone group is not present in this compound.
discussed the chapter Aldehydes and Ketones
The following points are discussed here
- 1. Introduction carbonyl Compounds
- 2. Nomenclature carbonyl Compounds
- 3. Structure of carbonyl Compounds
The compounds in which the -CO- group is attached to the alkyl group on both sides are also called Alkenones. The common molecule of these compounds is the formula CnH2nO, where n has a value greater than 2 or 2.
The general structure of these compounds is the formula R1-CO-R2, where R1 and R2 are the alkyl group. R1 and R2 may be the same or different. The two consecutive alkenones have a difference of CH2. Hence all alkenone are members of the same homogeneous category. The first member of this category is acetone (CH3-CO-CH3) and the second member is methyl ethyl ketone(CH3-CO-C2H5).
The carbonyl group is present in both aldehydes and ketones compounds, hence both these types of compounds are also called carbonyl compounds.
Nomenclature of Aldehydes
In the simple method, they are named by removing ek from the end of the common name of the corresponding acid and adding aldehyde.
In the IUPAC method, they are named by adding ‘al’ to the end of the corresponding alkane name. Remove ‘e’ from the end of the English name and add ‘al’. Since the alhydride group in alhydrides is located at one end of a series of carbon atoms, the number of carbon atoms associated with it is usually 1.
Therefore, it is often not necessary to display the number of carbon atoms belonging to the anhydride group in the IUPAC names of anhydrides.
Following are the common and IUPAC names of some anhydrides.
Nomenclature of Ketones
In the normal method, ketones are named based on the names of the groups associated with the ketone group.
In the IUPAC method, ketones are named by adding ‘one’ to the end of the corresponding alkane name. Removes ‘e’ from the end of the English name and adds ‘one’.
After this, the IUPAC name of that ketone is obtained after writing the appropriate and lowest number of carbon atoms related to ketone group in series of carbon atoms with the help of IUPAC rules.
Following are the common and IUPAC names of some ketones.
Isomerism in Aldehydes and Ketones
The aldehyde and ketone having the same molecule formula exhibit Functional Isomers.
Example: The molecule formula C3H6O exhibits the following two functional isomerism.
C2H5 – CHO → Propanal (Aldehyde)
CH3 – CO – CH3 → Propanal (Ketone)
Additionally ketone mainly exhibits Position Isomerism.
Example: 2-pentanone and 3-pentanone are isomerism instead of each other.
Additionally ketone mainly exhibits Position Isomerism.
Example: 2-pentanone and 3-pentanone are isomerism instead of each other.
CH3 – CO – CH2 – CH2 – CH3 → 2-pentanone
CH3 – CH2 – CO – CH2 – CH3 → 3-pentanone
In addition, aldehyde and ketone also exhibit chains isomerism.
Example: methyl n propyl ketone and methyl iso-propyl ketone are chain isomerism of each other.

Preparation of Aldehydes and Ketones
Oxidation of Alcohol: In the first step of oxidation of primary alcohols, aldehyde is obtained.
R – CH2OH + O → R – CHO + H2O
H – CH2OH + O → H – CHO + H2O
CH3 – CH2OH + O → CH3 – CHO + H2O
In the first term of oxidation of secondary alcohol, ketone is obtained.
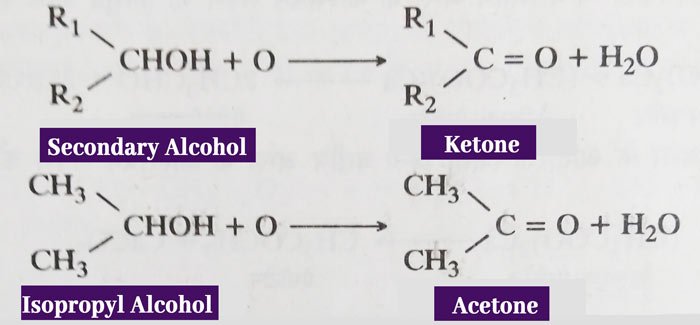
The above reactions can be carried out easily by acidic solution of Potassium Dichromate (K2Cr2O7). Products obtained from these reactions can be reacted with the oxidizer to form other products (Carboxylic Acid).
Hence, the amount of oxidizer, temperature and other conditions of reaction are arranged such that the main products are aldehydes or ketones, and other products are not obtained.
- Preparation of Aldehydes and Ketones : Chemistry Page
- Arsenious Oxide : Preparation, Properties and Uses
- How to find Equivalent Weight in Chemistry ? Chemical Formula
- Oxidation Number : How to find Oxidation State
- Bleaching Powder : Preparation, uses of Bleaching Powder
- Iodine : Properties, Preparation and uses
- Bromine – Preparation, Physical and Chemical Properties a Uses
- Chlorine Property : Physical and Chemical Properties | Uses
Dehydrogenation of Alcohol: The flow of primary alcohol vapors at 300°C on heated copper gives aldehyde. Under these conditions secondary alcohols is made ketones.
Aldehyde:
R – CH2OH → R – CHO + H2
H – CH2OH → H – CHO + H2
CH3 – CH2OH → CH3 – CHO + H2
Ketone:

By heating calcium salt of fatty acids: calcium salt of fatty acids can be made by the action of fatty acids and calcium hydroxide(Ca(OH)2).
Example:
2HCOOH + Ca(OH)2 → (HCOO)2Ca + 2H2O
2CH3COOH + Ca(OH)2 → (CH3COO)2 + 2H2O
Aldehydes or ketones can be made by dry distillation of calcium salt of fatty acids.
Aldehydes: formaldehyde is obtained from the dry distillation of calcium formate ((HCOO)2Ca).
(HCOO)2Ca → HCHO + CaCO3
Other aldehyde is obtained by distilling calcium salt of another fatty acid with calcium formate((HCOO)2Ca).
(HCOO)2Ca + (CH3COO)2Ca → 2CH3CHO + 2CaCO3
Ketones: Ketone is obtained by heating calcium salt of any other fatty acid other than formic acid.
(CH3COO)2Ca → CH3COCH3 + CaCO3
By passing fatty acid vapors heated to manganese oxide at 300°C:
Aldehyde: aldehyde is obtained from a mixture of formic acid and its homogeneous acids.
HCOOH + HCOOH → HCHO + CO2 + H2O
RCOOH + HCOOH → RCHO + CO2 + H2O
CH3COOH + HCOOH → CH3CHO + CO2 + H2O
Ketone: Other than formic acid, ketone is obtained from a mixture of other fatty acids.
R1 – COOH + R2 – COOH → R1 – CO – R2 + CO2 + H2O
CH3COOH + CH3COOH → CH3 – CO – CH3 + CO2 + H2O
Grignard reagent:
Aldehydes – aldehyde is obtained by reaction of Grignard reagent and ethyl formate in 1: 1 molecular ratio.
The alkyl (-R) group used in the above reaction is methyl (-CH3) group. That is, methyl magnesium halide (CH3MgCl) is reacted with methyl formate, then acetaldehyde (CH3CHO) is obtained as a product.
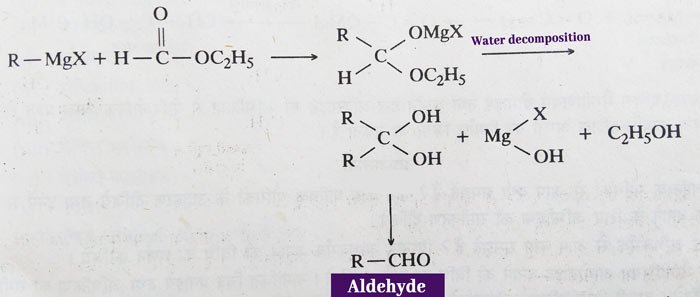
Ketone: In addition to formic acid, ketone is obtained by the reaction of esters of other acids and Grignard Reagent in a 1: 1 molecular ratio. In addition, ketone is also obtained from reactions with acid chlorides, acid amides and alkyl cyanides of the Grignard reagent.
CH3COCl + CH3MgCl → CH3COCH3 + MgCl2
CH3CONH2 + CH3MgCl → CH3COCH3 + Mg(NH2)Cl
Water decomposition of gem Dihalides:
Aldehyde: When both halogen atoms of gem dihalide are located on the carbon atom of the end, alhydride is obtained from their water decomposition.

Ketone: When both halogen atoms of gem dihalides are located on any other carbon atom other than the carbon atom at the end, ketone is obtained from their water decomposition.

Water decomposition of ozonides: The additive reaction of alkens and ozone results in ozonide. Water decomposition of ozonides yields aldehydes or ketones according to the structure of the alkene.
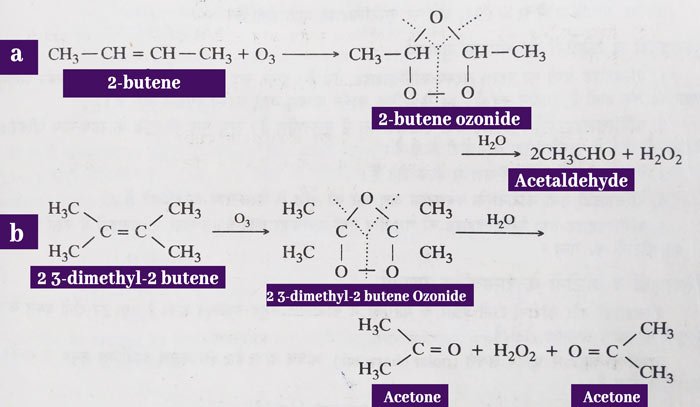
Water decomposition is done in the presence of zinc powder. This decomposes the hydrogen peroxide formed in the reaction. If hydrogen peroxide is not decomposed, it can oxidize the received aldehyde to acid.
Acetylene: Acetylene gas is formed when it is flushed into hot and dilute sulfuric acid in the presence of catalytic mercuric sulfate(HgSO4). The homogeneous form of acetylene forms ketone.
CH ≡ CH + H2O → CH3CHO
CH3 – C ≡ CH + H2O → CH3 – CO – CH3
By Rosenmund reaction: In the presence of Palladium-containing barium sulphate(BaSO4), acid chloride is reduced by hydrogen to form aldehyde. Ketone cannot be obtained by this method.
R – COCl + H2 → RCHO + HCl
CH3COCl + H2 → CH3 – CHO + HCl
Methods of Preparation of Aldehyde, Ketones
Rosenmund | Etard, Stephen, Gattermann-Koch Reaction
- a) Oxidation of alcohols
- b) Dehydrogenation of alcohols
- c) Ozonolysis of alkenes
- d) Hydrolysis of alkynes
Stephan’s method: On decomposition of ether dissolved alkyl cyanide with stannous chloride (SnCl₂) and hydrochloric acid, Imino chloride is obtained which decomposes water to form aldehyde. Ketone is not obtained by this method.
R – C ≡ N → R – CH = NH.HCl → R – CHO + NH4Cl
In the above reaction, Acetaldehyde is obtained when R = CH3.
Physical Properties
The first member of the aldehyde category is formaldehyde gas. Subsequent members of which the number of carbon atoms is up to 14, is a colorless liquid, the member having more than 14 carbon atoms is a colorless solid.
Formaldehyde, acetaldehyde and acetone are soluble in water. Water solubility of aldehydes and ketones decreases with increase in molecular weight
Aldehydes and ketones are lighter than water.
The boiling points of aldehydes and ketones increase with the increase of the molecular weight.
The smell of formaldehyde and acetaldehydes is strong and unpleasant. Higher aldehydes have a fruit-like odor. Fluid ketones have a sweet smell.
Chemical Properties
A carbonyl group of both aldehydes and ketones compounds is present and there is substantial similarity in the properties of both these compounds.
The carbonyl group has more polar character. This causes the carbon atom of the carbonyl group to be positively charged.
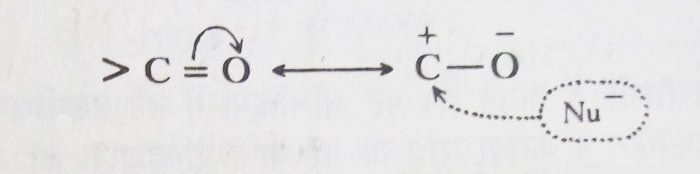
Most reactions of aldehydes and ketones occur due to the invasion of the nucleophile at the carbon atom of the carbonyl group. Therefore, the relative reactivity of aldehydes and ketones depends mainly on the amount of carbon atom of the carbonyl group.
If the amount of charge is high then the reactivity is high and if the amount of charge is low then the reactivity is low. On this basis, the decreasing order of action of formaldehyde, acetaldehyde and acetone is as follows.
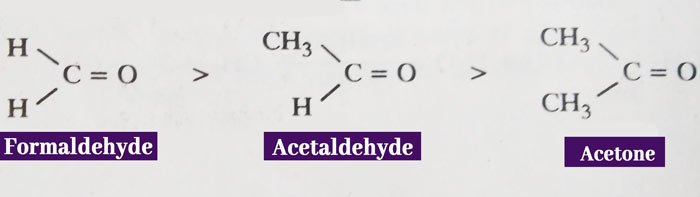
This is the electron releasing group due to the + I effect of the methyl group. Therefore, it reduces the carbon atom of the carbonyl group. Therefore, acetone has the lowest activity of the above three compounds.
The relative reactivity of aldehydes and ketones also depends on the size of the radicals associated with the carbonyl group. If the size of the radicals is large then the Nucleus lubricating reagent invasion of the carbon atom of the carbonyl group is difficult and the reactivity of the compound is low.
Addition Reaction
Addition of hydrogen: Aldehydes and ketones are reduced by reacting with hydrogen and primary and secondary alcohols are obtained respectively. This reaction can be performed by hydrogen and Ni, Na – Hg and water, Na – Hg and ethyl alcohol, LIAlH4 or NaBH4.
R–CHO + 2H → R–CH2OH
CH3 – CHO + 2H → CH3 – CH2OH
R – CO – R’ + 2H → R – CH(OH) – R’
CH3 – CO – CH3 + 2H → CH3 – CH(OH) – CH3
When an aldehyde or ketone is reduced with zinc mercury amalgam and concentrated HCl, the carbonyl group (-CO-) is converted into methyline (-CH2-) group. This reaction is called clemmensen’s reduction.
Example:
CH3 – CHO + 4H → CH3 – CH3 + H2O
CH3 – CO – CH3 + 4H → CH3 – CH2 – CH3 + H2O
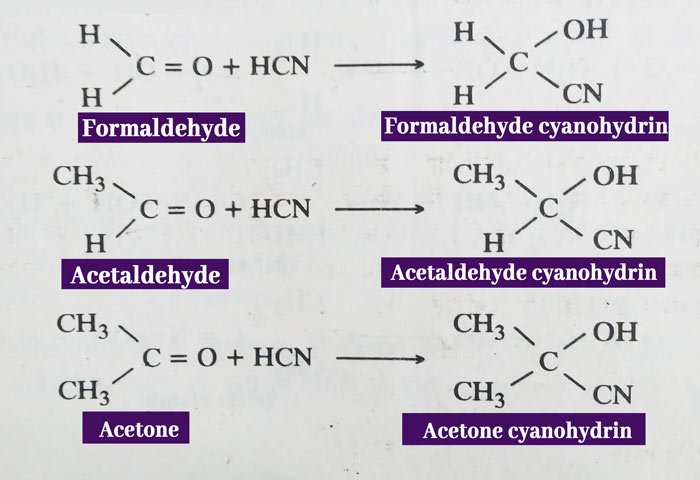
Addition of hydrogen cyanide: Aldehyde and ketone are reacted with hydrogen cyanide to form additive products called cyanohydrin.
Example:
Addition of sodium bisulfite: Aldehydes and ketones make crystal bisulfite compounds by adding sodium bisulfite(NaHSO3).

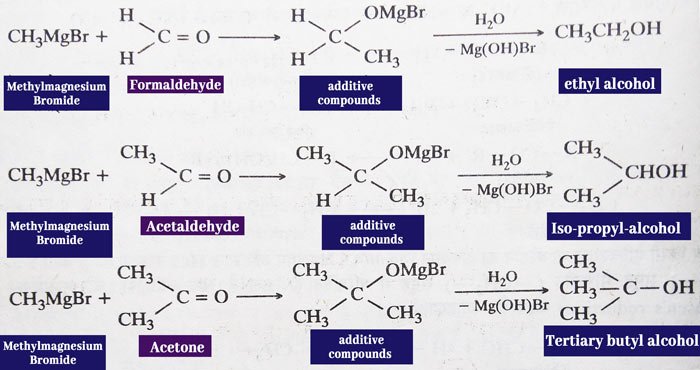
Addition of Grignard Reagent: aldehydes and ketones react
with the grignard reagent to form additive compounds which form alcohol
upon water decomposition. Primary alcohol is obtained from formaldehyde, secondary alcohol from all other aldehydes and tertiary alcohol from ketones.
Oxygen Replace in Carboxylic Acid
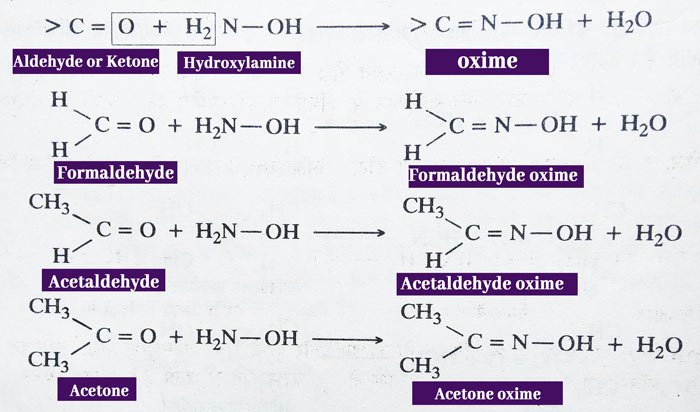
Reaction with Hydroxylamine: Aldehydes and ketones react with hydroxyl amines (NH2OH) to form crystal solids called oxime.
Reaction with Hydrazine: aldehydes and ketones react with hydrazine (NH2NH2) to form hydrazones.
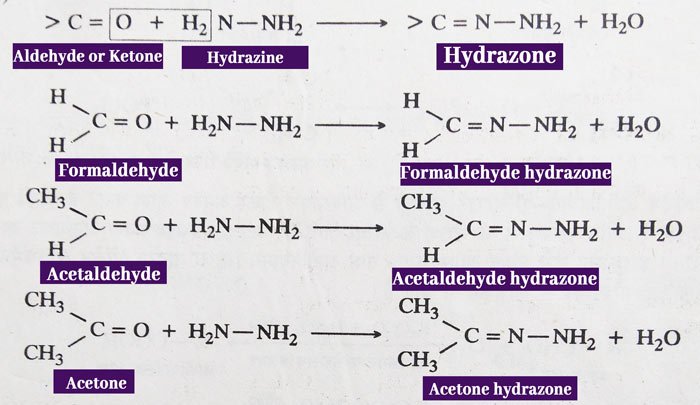
The reactions of aldehydes and ketones to phenyl hydrazine and 2,4-dinitrophenylhydrazine are similar.

2,4-dinitrophenylhydrazine is an orange-colored solid crystal substance and have a melting point. Therefore, the reaction of aldehydes and ketones with 2,4-dinitrophenylhydrazine is used to test and identify these compounds.
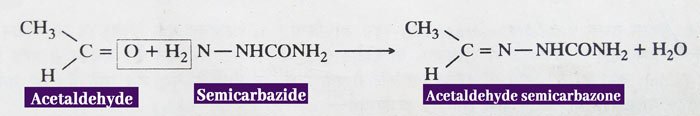
Reaction with Semicarbazide: Aldehydes and ketones react with semicarbazide to form semicarbanzone.
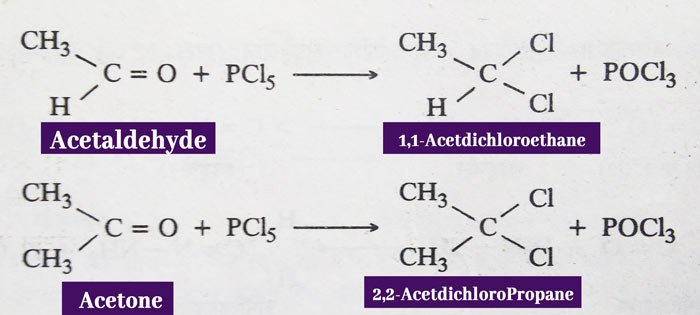
Phosphorus Pentachloride: In reaction with phosphorus pentachloride of aldehydes and ketones, the oxygen atom of the carbonyl group is displaced by two chlorine atoms.
Example:
Oxidation Reactions
The aldehyde is easily oxidized to form the corresponding acid, in which the number of carbon atoms is equal to the number of carbon atoms in the aldehyde. Hence, aldehyde acts as a strong reducing agent.
These acidic potassium dichromate are reacted with strong oxidants such as ammonium silver nitrate (tollens reagent: (Ag(NH3)2NO3)) and weak oxidants such as fahling solutions.

Ketone is not easily oxidized. These are oxidized to strong oxidizing agents such as acidic potassium dichromate to form acids in which the number of carbon atoms is less than the original additive. Ketone does not react with weak oxidants such as tollens reagent and fehling solutions.
R1 – CO – R2 → R1 – COOH + R2 – COOH
CH3 – CO – CH3 + 4O → CH3COOH + CO2 + H2O
Oxidation by selenium dioxide (SeO2) converts methylene group into carbonyl group adjacent to carbonyl group in aldehydes and ketones.
H – CH2 – CHO + SeO2 → H – CO – CHO + Se + H2O
H – CH2 – CO – CH3 + SeO2 → H – CO – CO – CH3 + Se + H2O
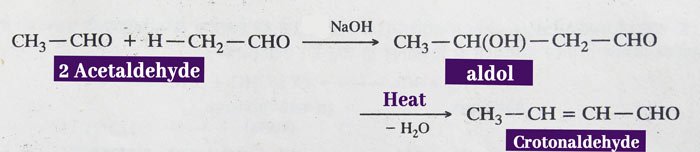
Aldol condensation: aldehyde or ketone containing α-hydrogen participate in this reaction. 2 molecules of aldehyde or ketone are condensed in the presence of dilute acid or base.
The product obtained from condensation is called an aldol. It has properties of both aldehyde and alcohol. An unsaturated aldehyde or ketone is obtained by heating it.
Aldehydes and Ketones
1. Cross-Aldol Reaction
2. Intramolecular aldol reaction
3. Questions & Answers for JEE/NEET
<iframe width="834" height="469" src="https://www.youtube.com/embed/cqQXhRcy4yc" title="Aldehyde and Ketones #10| Cross-Aldol Reaction| Intramolecular aldol reaction || important Questions" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share" referrerpolicy="strict-origin-when-cross-origin" allowfullscreen></iframe>
In the presence of dilute NaOH, two molecules of acetaldehyde are combined to form an aldol, which upon heating gives crotonaldehyde.
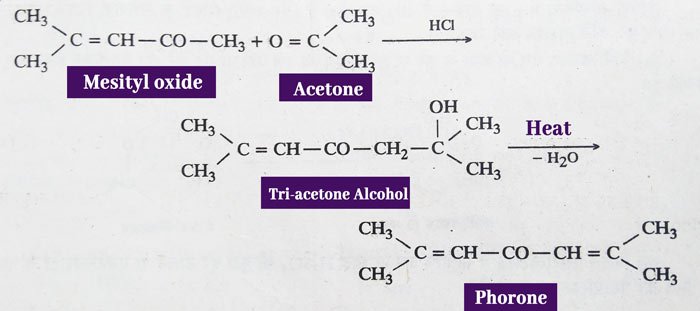
In the presence of barium hydroxide, condensation of two molecules produces di-acetone alcohol, which, on heating, gives mesityl oxide.

mesityl oxide and phorone are obtained from acetone in the presence of pure hydrogen chloride gas. Mesityl oxide is obtained from acetone according to the above reaction. Phorone is thus obtained from mesityl oxide.
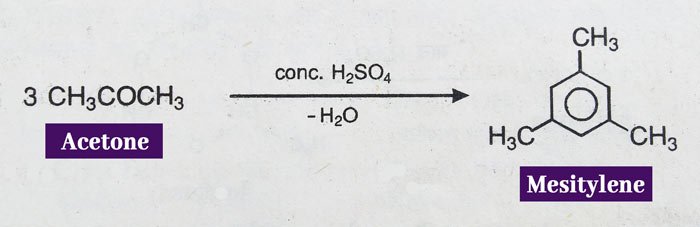
Mecetylene is formed when acetone is heated with concentrated H2SO4.
Halogenation
Reaction of aldehydes and ketones with halogens displaces hydrogen atoms of the alkyl group from halogen atoms.
CH3CHO + 3Cl2 → CCl3CHO + 3HCl
CH3 – CO – CH3 + 3Cl2 → CCl3 – CO – CH3 + 3HCl
Acetaldehyde and methyl ketone exhibit haloform reaction. The basis of this reaction is their above reaction. In the haloform reaction, the first hydrogen atoms of the methyl group attached to the carbonyl group are displaced by halogen atoms and then the alkali water decomposition results in the haloform.