Aluminium Element | what is Aluminium Toxicity | What Aluminium is Used For
Aluminium Ores
- Bauxite: Al2O3.2H2O
- Diaspore: Al2O3.H2O
- Corundum: Al2O3
- Felspar: K2O.Al2O3.6SiO2
- Mica: K2O.3Al2O3.6SiO2
- Kaolin: Al2O3.2SiO2.2H2O
- Alunite: K2SO4.Al2(SO4)3.4Al(OH)3
- Cryolite: Na3AlF6
- Fluorspar: CaF2
- Aluminium Hydroxide: Al(OH)3
- Aluminium Nitride: AlN
Here are
some common ores of aluminum along with their chemical formulas:
Bauxite: Bauxite
is the primary ore of aluminum and consists mainly of various aluminum minerals
along with impurities.
Chemical
Composition: Generally composed of hydrated aluminum oxides, with the main
minerals being gibbsite (Al(OH)₃), boehmite (γ-AlO(OH)), and diaspore
(α-AlO(OH)), along with other minerals like hematite, goethite, and quartz. The
overall formula is typically Al₂O₃·nH₂O.
Cryolite:
Cryolite is a rare mineral historically used in the production of aluminum as a
flux.
Chemical
Formula: Na₃AlF₆ (sodium aluminum fluoride)
These are
the primary ores from which aluminum is extracted. Bauxite, in particular, is
the main source of aluminum globally, while cryolite, although historically
significant, is less commonly used today due to its rarity.
Where is Aluminium Found
This element is not found in an independent state in nature. It is
found as different ores in the compound phase. In India it is found as
corundum in Rewa and Bhandar and as bauxite in Katni Balaghat Belgaum
Tamil Nadu Jammu Kashmir and Salem. Aluminum(8.1%) metal ranks third
among the most commonly found elements in the earth floor after
oxygen(49.2%) and silicon(25.7%).
The earth holds the first place among the metals found in the floor.
Aluminium-extraction factories in India are Aluminium Corporation of India JK Nagar West Bengal and the Indian Aluminium Company Limited Bihar Kerala Maharashtra Maharashtra and Madhya Pradesh.
Metallurgy of Aluminium
Bauxite(Al2O3.2H2O) is the major ore of aluminum. To obtain aluminium from bauxite ore, do the following steps sequentially.
Purification of the Bauxite Ore
Bauxite ore contains impurities of ferric oxide(Fe2O3) and silicon oxide(SiO2) etc. First of all remove them and get pure alumina. This process is called bauxite ore refining. Depending on the nature of the impurities, bauxite ore is treated by one of the following three methods.
Hall’s method
This method is used if impurities of Fe2O3 and SiO2 are present in approximately equal amounts in bauxite ore.
In this method, bauxite is melted by mixing ore with sodium carbonate.
Al2O3.2H2O + Na2CO3 → 2NaAlO2 + CO2 + 2H2O
The molten liquid is dissolved in water, soluble sodium metalluminate(NaAlO2) dissolves in water. By filtering the obtained mixture, impurities of ferric oxide and silicon oxide are separated.
The obtained aqueous solution is cooled down to 60°C and carbon dioxide gas flows. Due to which white precipitate of aluminum hydroxide is obtained.
2NaAlO2 + CO2 + 3H2O → Na2CO3 + 2Al(OH)3
Alumina is obtained by drying the obtained white precipitate in a furnace and heating it.
2Al(OH)3 → Al2O3 + 3H2O
Baeyer’s process
This method is used when the impurities of ferric oxide are high in bauxite and the impurities of feris oxide are also present.
In this method, the first is roasting of bauxite ore, which turns ferris oxide into ferric oxide. Then the impure bauxite is heated in an autoclave at 80 atmospheric pressure and 150°C heat with a solution of 45% caustic soda. By doing this, sodium meta aluminate is formed. The obtained mixture is filtered, which removes impurities of ferric oxide and silicon oxide.
Al2O3.2H2O + 2NaOH → 2NaAlO2 + 3H2O
Separate precipitated Al(OH)3 is added to the obtained solution and shaken well, which causes water decomposition of NaAlO2 by water in the presence of Al(OH)3 and white precipitate of aluminium hydroxide.
NaAlO2 + 2H2O → Al(OH)3 + NaOH
Alumina is obtained by drying the obtained white precipitate in an emitting furnace.
2Al(OH)3 → Al2O3 + 3H2O
Serpek’s Process
This method is used when the impurities of silicon are high in bauxite(Al2O3.2H2O).
In this method, bauxite is heated with carbon and nitrogen gas flows over the mixture. Which makes aluminium nitride. Silicon dioxide changes to silicon. Silicon evaporates and dissociates.
Al2O3.2H2O + 3C + N2 → 2AlN + 2H2O + 3CO
SiO2 + 2C → Si + 2CO
The obtained aluminium nitride is reacted with water, resulting in white precipitate of aluminium.
AlN + 3H2O → Al(OH)3 + NH3
Alumina is obtained by drying the obtained white precipitate in an emitting furnace.
2Al(OH)3 → Al2O3 + 3H2O
Electrolytic Decomposition of Alumina
Obtained pure aluminium metal from the alumina obtained by the above methods by valid decomposition method. This method is called hall heroult method.
In this method, for the legal decomposition of alumina, an iron vessel is used, with a coating of carbon on the inside walls. And this also serves as the cathode plate. The six anodes of the graphite acts as an anode.
A mixture of alumina and cryolite is filled in this iron vessel. It performs the function of electrolyte. To protect the eyes from glare and heat loss from radiation, we put coal powder over this mixture.
The temperature of the mixture is kept 875°-900°C in the vessel. A mixture of alumina and cryolite is melted at around 900°C. When current flows. So the following actions take place.

Ionisation of molten alumina occurs. Reduction of Al at the cathode gives Al metal.
Al2O3 ⇌ 2Al3+ + 3O2-
Al3+ + 3e → Al Cathode
O2- + 2e → O Anode
O + O → O2
4C + 3O2 → 2CO2 + 2CO
The role of cryolite is prominent in the electrical decomposition of alumina by the hall heroult method. The melting point of pure alumina is 2050°C. If a small amount of cryolite is added to the alumina, it begins to melt at low temperature (875°-900°C).
In the presence of cryolite the heat required for electrical decomposition is significantly reduced. And the cost of production comes down significantly. For this reason, the prices of aluminium were reduced considerably after the discovery of the hall heroult method The hall heroult method was discovered in 1885. In 1885 USA, the market price of aluminium was $ 100 per pound while in 1890 the market price of aluminium was $ 2 per pound.
Little Calcium fluoride(CaF2) can also be mixed with cryolite in alumina in the hall heroult method. Fluorspar (CaF2)also performs the same function as cryolite. That is, it reduces the melting point of the mixture and increases its electrical conductivity. In industrial processes, a mixture of 20 parts alumina, 60 parts cryolite and 20 parts CaF2 is often used.
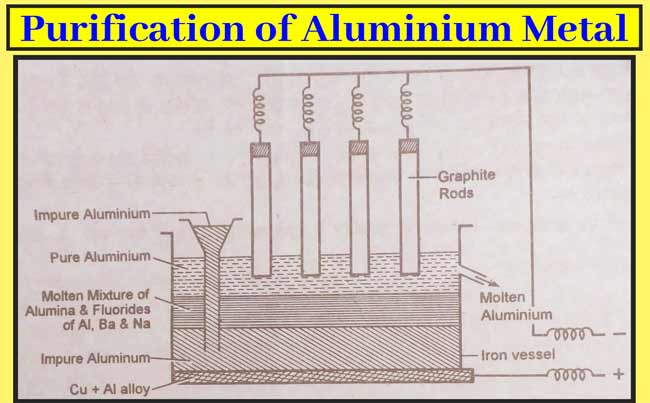
Purification of Aluminium Metal
The aluminium metal(99.00%) obtained from the above method is purified to 99.98% by the hoop’s method.
Aluminium uses an iron vessel to liquefy the metal. Which has an alloy of Cu and Al on its bottom, and acts as anode. The graphite plate serves as the cathode. There are three layers of molten material in the form of electrolyte. The lowest layer contains faux aluminium. In which there are impurities of silica etc. The middle part contains a mixture of aluminium sodium and barium fluoride and alumina. The topmost layer consists of pure aluminium which is in contact with the cathode.
When the current flows, the pure aluminium in the middle layer moves to the topmost layer and the same amount of aluminium from the bottom layer to the middle layer. This sequence goes on and pure aluminium continues to accumulate at the top. Which is taken out of the exit gate. 99.9% pure metals are obtained by this method.
Properties of Aluminium
Physical Properties
Aluminium has a metallic luster of white. It is electric and conductor of heat.
Its density is 2 grams per cubic cm.
Its melting point is 660°C.
Chemical Properties
Effect of air: Keeping aluminium metal in the air, a thin layer of aluminum oxide is applied on its surface. This layer acts as a protective layer. Aluminium does not rust due to the formation of a protective layer.
On heating aluminum metal in oxygen, water rises and aluminum oxide is obtained.
4Al + 3O2 → 2Al2O3
Aluminium oxide is an amphoteric oxide and reacts with both acids and bases.
Al2O3 + 6HCl → 2AlCl3 + 3H2O
Al2O3 + 2NaOH → 2NaAlO2 + H2O
Effect of water: Pure aluminium does not react with water.
Reaction with Acids: (A) Aluminium dissolves in dilute and concentrated HCl to form aluminium chloride and liberates hydrogen gas.
2Al + 6HCl → 2AlCl3 + 3H2
By evaporating the obtained solution, crystals of hydrogen aluminium chloride (AlCl3.6H2O) are obtained.
(B) Aluminium dissolves in dilute H2SO4 to form aluminium sulphate and liberates hydrogen gas.
2Al + 3H2SO4 → Al2(SO4)3 + 3H2
Evaporation of the obtained solution gives crystals of hydrogen aluminium sulfate.
Sulfur dioxide is obtained when aluminum is heated with concentrated H2SO4.
2Al + 6H2SO4 → Al2(SO4)3 + 6H2O + 3SO2
(C) Due to surface oxidation by nitric acid, an impermeable oxide film is formed on its surface and it becomes passive. Hence nitric acid has no action on aluminium metal.
Reaction with caustic soda: It dissolves in concentrated caustic soda solution to form sodium Meta aluminate and liberates hydrogen gas.
2Al + 2NaOH + 2H2O → 3H2 + 2NaAlO2
Reaction with halogens: Anhydrous aluminium chloride or anhydrous aluminium bromide is obtained by heating dry chlorine or bromine gas in hot aluminium.
2Al + 3Cl2 → 2AlCl3
2Al + 3Br2 → 2AlBr3
Reaction with Nitrogen: Aluminium combines with nitrogen to make aluminium nitride.
2Al + N2 → 2AlN
The obtained aluminium nitride reacts with water to make white precipitate of aluminium hydroxide and ammonia gas is released.
AlN + 3H2O → Al(OH)3 + NH3
Aluminium uses
Aluminium is a light metal with no corrosion. Therefore, it is used in making aluminium sheets, utensils, airplanes and motors etc.
Aluminium wires are used for flow of electricity.
Aluminium foils are used for wrapping cigarette soap etc.
Aluminium is used in the extraction of chromium and manganese.
Aluminium is used in thermit welding.
Aluminium alloys are useful.
