Amines: Nomenclature, Isomerism, Basic Characters
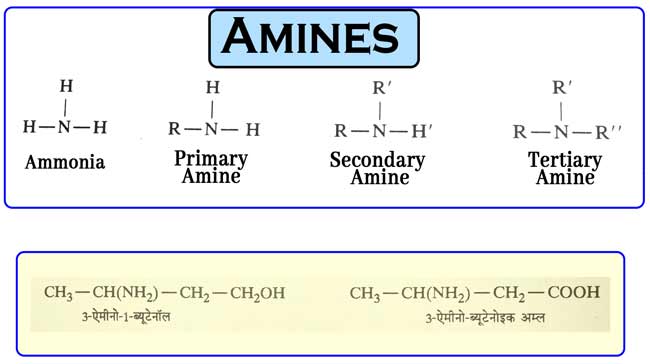
The alkyl derivatives of ammonia are called amine. In other words, the compounds that are obtained when the hydrogen atoms in the ammonia molecule are replaced by an equal number of alkyl groups. They are called amine.
They are divided into three classes based on the number of alkyl groups associated with the
nitrogen atom in amines.
The primary amines have only one alkyl group attached to the nitrogen atom, the secondary amines have two alkyl groups attached to the nitrogen atom and the tertiary amines have three alkyl groups attached to the nitrogen atom.

The alkyl group present in secondary and tertiary amines can be the same or different. These structures represent R, R ‘, R’ ‘alkyl group.
Those other compounds are also called amine, In which nitrogen atom is attached to some other groups but their properties are similar to the properties of these compounds. Example:-
Aniline(C6H5NH2) is also a primary Amin. Similarly, Cyclohexylamine (C6H13N) and Diphenylamine (C12H11N) are also amine compounds.
Nomenclature of Amines
In the simplest method, amine is named based on the name of the alkyl group combined with the nitrogen atom. To write the common name of an amine, we append the attached amine to the end of the alkyl group name.
If two or three different alkyl groups are present in amine, then the name of the small alkyl group is written first and the name of the large alkyl group is written later.
- Amines: Nomenclature, Isomerism, Basic Characters
- How is Ethyl Acetate made? | Properties | Uses
- Is Acetamide base or acid? | Preparation | Properties | Uses
- Acetic Anhydride: What is acetic anhydride used for?| Preparation | Properties
- Acetyl chloride: How do you make Acetyl Chloride? | Properties | Uses
- Carboxylic Acid: What is the common name of a carboxylic acid?
- Oxalic Acid: What is the formula of oxalic acid? | Properties | Uses
In the IUPAC method, amines are named based on the name of the corresponding alkane. To write the IUPAC name of the primary amines, append the appended amine to the end of the corresponding alkane name, write the entire name of the compound as a single word and display the carbon atom carrying the amine group by the appropriate number.
The IUPAC names of secondary and tertiary amines are written in a similar way, but N – alkyl and N also use N–di alkyl precursors to indicate the presence of other alkyl groups.
Following are the common and IUPAC names of some amines.
If the amines group present in a compound is not the predominant functional group, then for this, pre-existing amines are used.

Isomerism of Amines
Amines compounds mainly exhibit three types of isomerism.
Chain isomerism
This type of isomerism is caused by the differing chain structure of carbon atoms.

Position Isomerism
This type of isomerism occurs because of the location of the amines group in the chain of carbon atoms.

Metamerism
This type of isomerism is caused by the variation of the size of the groups connected on both sides of the same nitrogen atom.

The basic Character of Amines
The basic salt of ammonia is caused by the lone pair of electrons on nitrogen. The presence of lone pair of electrons causes one molecule of ammonia (NH3) to accept a proton (H+) and form ammonium ion(NH4+).
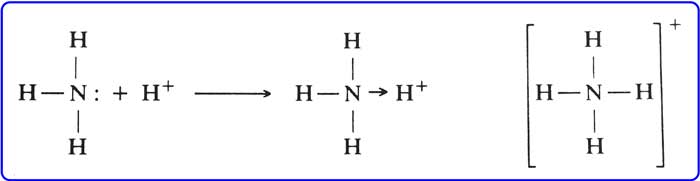
Like ammonia, lone pair of electrons are present on the nitrogen atom and amines compounds are also basic. Due to the + I effect of alkyl groups, they displace electrons from themselves.
Hence the availability of lone pair of electron on nitrogen atoms in amines increases. Therefore, aliphatic amines are more potent than compound ammonia.

On this basis, tertiary amine should be the most incinerate and in decreasing order of Sentimentalism, it will be followed by secondary amine, primary amine and ammonia. The decreasing order of the aseptic strength of these compounds is often in this way.
The sequence of incineration of secondary and tertiary amines is not consistent with the induction effect. This can be explained on the basis of hindrance in the reaction due to steric effect i.e. presence of large size groups near the reaction center.
The following is the decreasing order of some amines carbonic compounds and the strength of ammonia.
- (CH3)2NH > CH3NH2 > (CH3)3N > NH3
- (C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3
- CH3 > C6H5N(CH3)2 > C6H5NHCH3 > C6H5NH2
Due to the basic properties of amines compounds, they react with acids to form salts. These salts are alkyl derivatives of ammonium salts. Salts in which nitrogen atom is attached to four alkyl group. And forms a legitimate connector. They are called quarternary ammonium salt.
Examples:
[(CH3)4N+]Cl–
[(CH3)4N+]OH–