Ammonia Gas : Preparation and Properties
Chemical Formula
- Calcium cyanamide- CaCN2
- Magnesium Nitride:- Mg3N2
- Sodium zincate:- Na2Zn(OH)4
- Diamine Silver Chloride: [Ag(NH3)2Cl]
- Silver Nitrate: AgNO3
- Mercurous Chloride:- Hg2Cl2
- Calcium cyanamide:- CaCN2
- Urea:- NH2CONH2
Ammonia gas was first obtained by Joseph Priestley in 1771 by heating the ammonium and lime together and named it Alkaline Air.
In some quantities it is found in the atmosphere. In places where animals rot, its quantity is more than expected in the air. In nature it is also found as its salts.
Example:
In nature, it is found as ammonium chloride (NH4Cl) and ammonium sulphate [(NH4)2SO4].
Preparation Normal Methods
Ammonization salts: Ammonia is obtained by heating ammonium salts with alkalis. Some ammonium salts give ammonia only on heating alone.
2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3
(NH4)2SO4 + 2NaOH → Na2SO4 + 2H2O + 2NH3
(NH4)2SO4 → H2SO4 + 2NH3
Addition of Nitrogen and Hydrogen: Ammonia is obtained by adding nitrogen and hydrogen at about 500°C temperature and 200 atmospheric pressure in the presence of
catalyst (Iron).
N2 + 3H2 → 2NH3
From Calcium Cyanamide: Ammonia is obtained by the action of water vapor on calcium
cyanamide.
CaCN2 + 3H2O → 2NH3 + CaCO3
By the action of water on Magnesium Nitride:
Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3
From Metallic Nitrate: Ammonia gas is obtained by the reaction of metallic nitrate with nascent hydrogen. The nascent hydrogen used in this activity is obtained by heating zinc or aluminum with sodium hydroxide.
4Zn + 7NaOH + NaNO3 → 4Na2ZnO2 + 2H2O + NH3
From Carbonic Compounds: When some nitrogen containing organic compounds are heated with soda lime, ammonia is obtained.
NH2CONH2 + NaOH → Na2CO3 + 2NH3
Preparation in Laboratory
In the laboratory, ammonia is made by heating the ammonium chloride with a quenched lime.
2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3
In a rigid glass tube, a mixture of ammonium and quenched lime is heated. The received ammonia gas is collected from the gas jar by downward displacement of air.
Before being collected in a gas jar, it is passed onto CaO, which removes the moisture of this gas.

Note: Ammonia cannot be dried by concentrated sulfuric acid H2SO4, calcium chloride, phosphorus penta oxide because ammonia undergoes chemical reactions with these compounds.
2NH3 + H2SO4 → (NH4)2SO4
2NH3 + CaCl2 + 2H2O → 2NH4Cl + Ca(OH)2
6NH3 + P2O5 + 3H2O → 2(NH4)3PO4
Industrial Manufacture
The industrial manufacture of ammonia is done by the following methods:
Haber's Process
In this method, nitrogen and hydrogen combine to form ammonia gas.
N2 + 3H2 ⇌ 2NH3 + 24,000 calorie
In this activity, heat is generated and heat is increased. This is an exothermic reversible reaction. In this reaction the volume of the reactants decreases.
According to the principle of le-chatelier, it is necessary to have low temperature and high pressure in this action. In this way more ammonia can be obtained and the action
velocity can be increased.
The presence of suitable catalysts also helps in increasing the velocity of action. But the optimum temperature of the reaction is about 500°C in the presence of the catalyst used in the reaction.
Therefore, in this reaction the temperature is kept at around 500°C. The pressure value is kept equal to about 200 atmospheres.
In this method any of the following catalysts can be used –
- Iron granular powder containing a small amount of malibdanam
- Ferric oxide containing soda, silica and potassium
- Fine powder of nickel

In the industrial manufacture of ammonia by the Haber method, a mixture of iron and molybdenum is often used as a catalyst. Molybdenum catalyst acts as a promotor.
Nitrogen gas obtained from the atmosphere and hydrogen from the water gas are mixed in dry state in a ratio of 1: 3 and flow into a chamber at 200 atmospheric pressure, where the catalyst is kept at around 500°C.
In this chamber, both these gases are reacted together to produce ammonia. The remaining gases are re-circulated in this chamber by a circulatory pump.
Cyanamide Process
A mixture of CaCN2 and carbon is obtained when CaC2 and nitrogen are heated to almost 1100°C temperature. Also called Nitrolime and which is also used as fertilizer.
CaC2 + N2 → CaCN2 + C
Ammonia gas is obtained by its reaction at 180°C and 3-4 atmospheric pressure with water vapor.
CaCN2 + 3H2O → CaCO3 + 2NH3↑
Manufacture of Coal Gas
In the manufacture of coal gas
As a side product, ammonia-rich liquor is obtained when making coal gas, which contains ammonium salts in large quantities. Ammonia gas is obtained by boiling it with lime water.
Physical Properties
It is a colorless and intensely smelling gas. Due to this, tears start coming out of the eyes. This can also be fatal due to an overdose.
The molecular weight of ammonia is 17 and density is 8.5. Hence it is lighter than air. This is why in the laboratory it is collected by downward displacement of air.
It is soluble in water. It also dissolves in large quantities in water. At normal temperature, its water content is 89.9 grams per 100 ml. Like other gases, its solubility in water decreases at higher temperatures.
Ammonia dissolves in water to form ammonium hydrosidae –
NH3 + H2O → NH4OH ⇌ NH4+ + OH–
Ammonium hydrosidae is a valid additive. Hence, aqueous solution of ammonia is alkaline and conductor of electricity.
Ammonia gas does not burn itself nor is it helpful in burning other substances. It does not burn when ammonia gas is ignited in the air. But it starts burning due to excess of oxygen. She extinguishes when a burning candle is carried in a jar of ammonia gas.
The critical temperature of ammonia is 133°C. Therefore, it keeps on increasing pressure at normal temperature.
The boiling point of liquid ammonia is -33.4°C and freezing point -77.7°C. Ammonia has a higher boiling point than phosphine(PH3). The reason for this is that the negative efficiency of nitrogen is higher than the negative efficiency of phosphorus. Hence intermolecular hydrogen bonding occurs in ammonia. And more energy is required to separate its molecules.
Chemical Properties
Reaction with water: Ammonia dissolves in water to form ammonium hydroxide-
NH3 + H2O → NH4OH ⇌ NH4+ + OH–
Alkaline properties: It reacts with acids to form salts.
2NH3 + H2SO4 → (NH4)2SO4
NH3 + HCl → NH4Cl
NH3 + HNO3 → NH4NO3
Combustion: Ammonia does not burn itself or helps in the burning of other substances. It burns with yellow flame in excess of oxygen.
4NH3 + 3O2 → 6H2O + 2N2
Nitric oxide and water are obtained when a mixture of ammonia and oxygen gas flows through a hot plate of platinum.
4NH3 + 5O2 → 6H2O + 4NO
Thermal Decomposition: Ammonia is a permanent additive but dissociates into components at high temperatures or when electric sparks flow.
2NH3 → N2 + 3H2
Action with halogens: If more amount of ammonia is taken with less amount of chlorine, nitrogen gas is obtained.
2NH3 +
3Cl2 → N2 + 6HCl
6NH3 +
6HCl → 6NH4Cl
8NH3 +
3Cl2 → N2 + 6NH4Cl
If an excess amount of chlorine is treated with a low amount of ammonia, then nitrogen tri-chloride is formed, which is a highly explosive substance.
3Cl2 + NH3 → NCl3 + 3HCl
Ammonia has similar action with bromine and iodine.
Reaction with Magnesium: It reacts with magnesium metal at high temperature to form magnesium nitride (Mg3N2).
3Mg + 2NH3 → Mg3N2 + 3H2
Action with alkaline metals: It reacts with alkaline metals at high temperature to form amide[Sodium amide (NaNH2)].
2Na + 2NH3 → 2NaNH2 + H2
Action with copper oxide: It is oxidized to nitrogen on red hot copper oxide.
2CuO + 2NH3 → 3Cu + 3H2O + N2
Its aqueous solution (NH4OH) combines with some salts to form complex compounds.
1) When a white precipitate of silver chloride (AgCl) is added in large amount with aqueous solution of ammonia, ie ammonium (NH4OH), dissolves due to the formation of complex compounds called diamine silver chloride[Ag(NH3)2Cl].
AgCl + 2NH4OH → [Ag(NH3)2]Cl + 2H2O
2) Aqueous solution of copper sulfate (CuSO4) is blue due to Cu++ ion. It forms Copper hydroxide Cu(OH)2 by first passing ammonia or adding an aqueous solution of ammonia.
Copper hydroxide is insoluble in water and its color is greenish blue. Therefore, greenish blue precipitate is obtained.
A dark blue solution is obtained when a large amount of ammonium hydroxide is flowed or an excess amount of ammonium hydroxide is formed by the formation of complex compounds called tetra amine cupric sulphate. The thick blue color of tetra amine cupric sulphate is due to Cu(NH3)4++ ion.
CuSO4 + 4NH4OH → Zn(NH3)4Cl2 + 4H2O
3) A white precipitate of zinc hydroxide is obtained when ammonium hydroxide solution is added to aqueous solution of zinc chloride(ZnCl2). It dissolves a large amount of ammonium hydroxide to form a complex compound called tetra amine zinc chloride[Zn(NH3)4Cl2] which is merged with water.
ZnCl2 + 4NH4OH → Zn(NH3)4Cl2 + 4H2O
Anhydrous zinc chloride also absorbs the ammonia gas to make this complex compound.
ZnCl2 + 4NH3 → Zn(NH3)4Cl2
Reaction with Silver Nitrate: Aqueous solution of ammonia reacts with silver nitrate (AgNO3) to form a white brown precipitate of silver oxide(Ag2O) which dissolves in excess of ammonium to form a merged complex salt. The solution thus obtained is called tollen’s reagent.
2AgNO3 + 2NH4OH → 2NH4NO3 + H2O + Ag2O
Ag2O + 2NH4NO3 + 2NH4OH → 2[Ag(NH3)2]NO3 + 2H2O
Reaction with mercurous chloride: A dilute aqueous solution of ammonia in mercuric chloride solution forms a mixture of mercury and mercuric amino chloride, which is black in color.
Hg2Cl2 + 2NH4OH → Hg + Hg(NH2)Cl + 2H2O + NH4Cl
Reaction with Mercuric Chloride: White precipitate of mercuric amino chloride is obtained by adding aqueous solution of ammonia to mercuric chloride solution.
HgCl2 + 2NH4OH → NH4Cl + 2H2O + Hg(NH2)Cl
Action with Nessler’s reagent: The alkaline solution of Potassium tetraiodomercurate(II) is called Nessler’s reagent. On reaction with ammonia, Iodide of Millon’s base and a brown precipitate is obtained. This reaction is also used to test ammonia and ammonium ions.
2K2HgI4 + 3NaOH + NH3 → 4KI + 3NaI + 2H2O + HgO⋅Hg(NH2)I↓
Uses of Ammonia
- It is used as a reagent in the laboratory.
- In the industrial manufacture of nitric acid
- In making urea, ammonium salts and fertilizers.
- Artificial silk tear gas is also used in making explosive material, ice, etc.
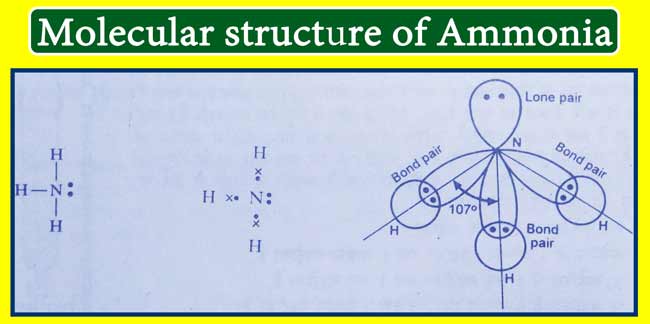
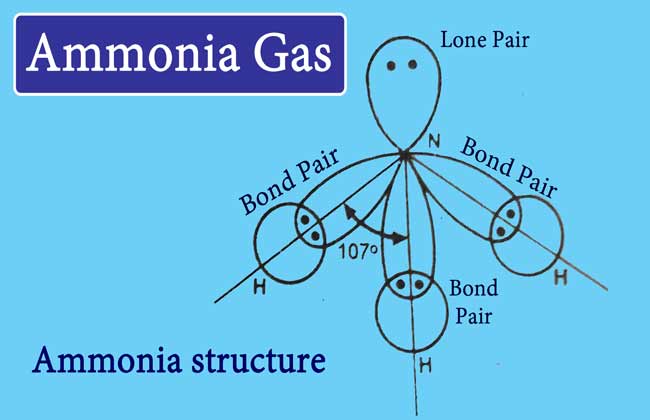
Molecular structure of ammonia
Following is the bond formula, electron point formula and orbital diagram of ammonia-
The hybridization of nitrogen atom in ammonia is of the sp3 type and has a pyramidal shape. The Basic property of ammonia is due to the lone pair of electrons present on nitrogen in it.