What is aniline used for? Preparation, Properties, and Tests
Aniline Preparation
Laboratory method: from tin and HCl reduction of nitrobenzene: in the laboratory, aniline is made by the reduction of nitrobenzene by tin and HCl.
Sn + 4HCl → SnCl4 + 4H
C6H5NO2 + 6H → C6H5NH2 + 2H2O
The aniline thus obtained combines with stannic chloride and hydrochloric acid to form a double salt. On adding sodium hydroxide solution to the mixture, the salt is doubly reduced. Aniline is obtained by fresh distillation of the obtained mixture.
To make aniline in the laboratory, take 25 grams of nitrobenzene and 50 grams of granulated tin in a round flask. A vertical air condenser is to be placed in the flask. Add 110 ml of concentrated hydrochloric acid little by little to the flask.
While adding acid, keep the flask shaken and cool so that the tap of the mixture does not exceed 60°C. When the whole acid is mixed, then keep the flask on water-heat for about half an hour, so that the reaction is complete.
Now after cooling the flask, a concentrated solution of sodium di-hydroxide is added to it, the result of which is separated as an unilinear fluid. Aniline is to be separated from this mixture by fresh distillation. Aniline is to be extracted by ether from the mixture of aniline and water obtained.
Why Aniline is less basic than Methyl Amines and Ammonia
Topic covered 1. Basicity order of Amines (1°, 2°, 3°) 2. Comparison of basicity of Amines with Aniline and Ammonia
<iframe width="834" height="469" src="https://www.youtube.com/embed/ZmaHRUhRUso" title="Why Aniline is less basic than Methyl Amines and Ammonia: Basic order of Amines (Pri, Sec, Ter)" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share" referrerpolicy="strict-origin-when-cross-origin" allowfullscreen></iframe>
The small amount of water present in the ether solution is drained away by burning potas and it is removed. From the solution obtained, the ether is separated by distillation, the pure aniline is obtained in the fraction obtained at 183-185°C.
Industrial manufacturing: from iron and HCl reduction of nitrobenzene: Aniline is made by reduction of nitrobenzene by moist iron shavings and HCl in commercial quantities.
Fe + 3HCl → FeCl3 + 3H
C6H5NO2 + 6H → C6H5NH2 + 2H2O
Chlorobenzene: Chlorobenzene gets aniline at high pressure with ammonia in the presence of Cu2O and when heated to about 250°C.
C6H5Cl + NH3 → C6H5NH2 + HCl
Phenol: phenol gives aniline at high pressure and heated to 300°C with ammonia in the presence of anhydrous ZnCl2.
C6H5OH + NH3 → C6H5NH2 + H2O
Benzamide: The amide (-CONH2) group is modified into the amines (-NH2) group upon heating amides with bromine and caustic potash. This reaction is called the Huffman bromide reaction. This reaction is used to make aniline from benzamide.
C6H5CONH2 + Br2 + 4KOH → C6H5NH2 + K2CO3 + 2KBr + 2H2O
Aniline Physical Properties
In pure state, aniline is a colorless liquid. Its boiling point is 184°C. When exposed to air, it gets oxidized in small amounts and due to this its color becomes light brown. It is less soluble in water but more soluble in ether, alcohol and chloroform. Its relative density is 1.02. It has a distinct smell. It is a toxic substance.
Chemical Properties
Chemical reactions of aniline can be divided into three parts.
- Reactions of amines group which is similar to aliphatic amines.
- Reactions of amines group which is different from aliphatic amines.
- Replacement reactions of nuclei
Basic character: Similar to aliphatic amines, aniline also reacts with acids to form salts.
Example:
C6H5NH2 + HCl → C6H5NH3+Cl–
This is a weaker base than ammonia and aliphatic amines. The reason for this is that the amines group in aniline is linked to the phenol group and the phenol group is negative. The availability of a lone electron pair on nitrogen is reduced due to the -I effect of phenol group and the + M effect of amines group.
Hence
aniline is a weaker base than ammonia and aliphatic amines such as CH3NH2
and C2H5NH2. In benzene ring, its basic
increases in presence of positive electric group. On this basis, ortho meta and
para Toluidine have a stronger base than aniline.
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
- What is Urea || How to make Urea Fertilizer, || Urea uses
Alkylation: This secondary amin reacts with alkyl halides to form tertiary amine and quaternary ammonium compound.
C6H5NH2 + CH3Cl → C6H5NHCH3 + HCl
C6H5NHCH3 + CH3Cl → C6H5N(CH3)2 + HCl
C6H5N(CH3)2 + CH3Cl → [C6H5N(CH3)3]+Cl–
Acetylation: The reaction of aniline with acetyl chloride or acetic anhydride results in acetylation. In this reaction, a hydrogen atom of the amines group of aniline is displaced by an acetyl (CH3CO-) group.
C6H5NH2 + CH3COCl → C6H5NHCOCH3 + HCl
C6H5NH2 + (CH3CO)2O → C6H5NHCOCH3 + CH3COOH
Reaction of aniline with benzoil chloride (C6H5COCl) results in its benzoylation, similar to acetylation, and benzanilide(C6H5NHCOC6H5) is obtained.
Carbylamine Reaction: Like alifatic amines, aniline also exhibits a carbylamine reaction. On heating it with chloroform and NaOH, a highly deodorant phenyl iso cyanide(C6H5NC) is formed.
C6H5NH2 + CHCl3 + 3KOH → C6H5NC + 3KCl + 3H2O
Grignard reagent reaction: It reacts with Grignard reagents to form hydrocarbon.
C6H5NH2 + CH3MgBr → CH4 + C6H5NHMgBr
Reaction with aldehydes: By reacting with aldehydes, this imines form common names, also known as schiff bases.
C6H5NH2 + C6H5CHO → C6H5 – N = CH – C6H5 + H2O
Reactions of amines group which is different from alifetic amines.
Reaction with nitrous acid: reacts with aniline nitrous acid in the presence of hydrochloric acid to form benzene di azonium chloride. The nitrous acid required for this reaction is obtained by the action of NaNO2 and HCl. This reaction is carried out at low temperature (0 – 5°C) because the benzene di azonium chloride decomposes at higher temperatures. In this reaction, -NH2 group is converted into -N group. This reaction is called diazotisation.
C6H5NH2 + HNO2 + HCl → C6H5 – N = N – Cl + 2H2O
The benzene diazonium chloride present – N = N – Cl group can be easily converted into other groups. Hence this reaction is used to make many compounds from aniline to benzene, chlorobenzene, phenol etc. benzene diazonium chloride exhibits coupling reaction with beta naphthyl and some other compounds in alkaline solutions.
Dyes are obtained as a result of this reaction. This reaction is used to test aniline and to make pigments.
- What is aniline used for? Preparation, Properties, and Tests
- How do you make Nitrobenzene? | Properties, Tests, and uses
- What is the formula of Chlorobenzene? Preparation, Properties and uses
- NEET 2021 / JEE 2021 Crash Course for Chemistry Subject
- What do you mean by Benzene? Preparation and Properties
- Why are aromatic compounds called aromatic? | Definition, Properties
- What is toluene used for? Preparation and Properties
- Ethyl Amine: Preparation, Properties, Uses, and Tests
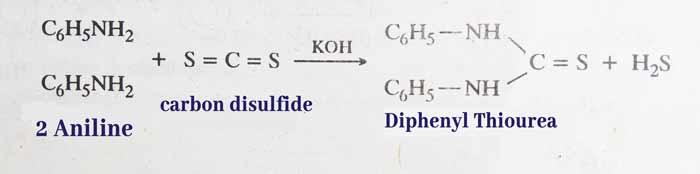
Reaction with carbon disulphide: It reacts with carbon disulfide in alcohol solution in the presence of solid KOH to form di phenyl thio urea.
Oxidation: Aniline is readily oxidized. Acidic potassium is oxidized by dicromate to form a para benzoquinone.

It is oxidized by hydrogen peraoxide in the presence of trifluoro acitic acid to form nitrobenzene. It is oxidized by hypoclorus acid (HClO) to form pera amines phenol. It is azobenzene oxidized by alkaline KMnO4. It is oxidized by acidic KMnO4 to form a dye called aniline black.
Replacement reactions of nuclei
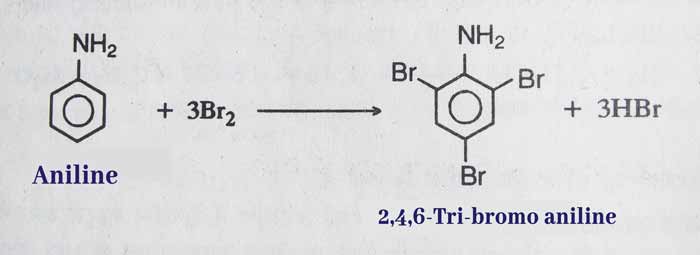
Halogenation: halogenation of aniline occurs at extremely rapid speeds. On adding bromine to aniline, white precipitate of 2,4,6-Tri-bromo aniline is obtained immediately.
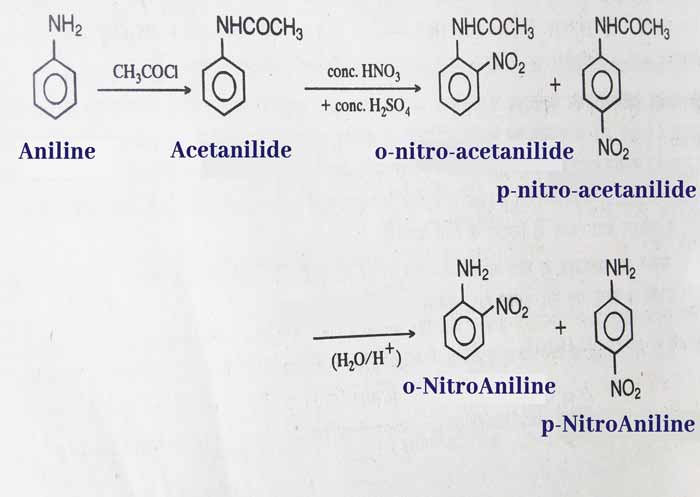
Nitration: The reaction of aniline with a mixture of concentrated NHO3 and concentrated H2SO4 does not lead to nitration. This is because aniline is oxidized by nitric acid. To nitration of aniline, first protect the amines group.
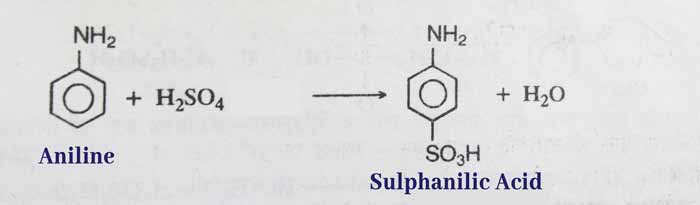
Sulphonation: The main product is sulphanilic acid when aniline is reacted with sulphuric acid.
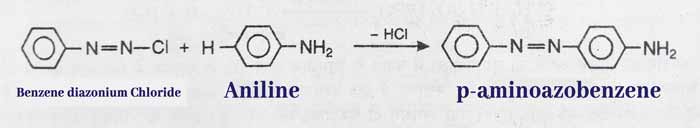
Coupling Reaction: It reacts with benzene di azonium chloride to form p-aminoazobenzene which is a dye and has a Orange red color.
Aniline uses
Aniline is used as a catalyst in the manufacture of other aromatic compounds, in the manufacture of pigments, in the manufacture of drugs, and in vulcanisation in rubber industry.
Aniline Tests
By mixing chloroform and alcohol KOH solution in aniline, a very deodorant substance is formed upon heating.
Benzene di azonium chloride is formed when aniline combines sodium nitrite and hydrochloric acid at 0 – 5°C temperature. A bright red color or precipitate is obtained by adding beta naphthol solution made in NaOH to the mixture obtained.
When adding bromine to dilute aqueous solution of aniline, white precipitate is obtained immediately.