The Chemistry of Aromatic Hydrocarbons: Understanding Stability and Reactivity
Aromatic hydrocarbons, also known as arenes, are a significant class of organic compounds characterized by their stable ring structure and unique chemical properties. The most well-known member of this class is benzene (C₆H₆), a simple aromatic compound with a six-carbon ring and alternating double bonds.

This discussion will delve into the structure, properties, synthesis, reactions, and applications of aromatic hydrocarbons, providing a comprehensive overview of their importance in chemistry and industry.
What is the specific structure of Benzene?
Based on the data of studies and calculations, it was concluded that all six carbon atoms are in the state of sp2 hybridization and lie in the same plane. Non-hybridized p-orbitals of carbon atoms constituting double bonds (Kekule formula) are perpendicular to the plane of the ring and parallel to each other.

They overlap with each other, forming a single π-system. Thus, the system of alternating double bonds depicted in the Kekule formula is a cyclic system of conjugated, overlapping π-bonds. This system consists of two toroidal (donut-like) regions of electron density lying on both sides of the benzene ring. So, to represent benzene in the form of a regular hexagon with a circle in the center (π-system) is more logical than in the form of cyclohexantriene-1,3,5.
that is, to consider it as an intermediate compound, “averaging” of two structures.
Measurements of bond lengths confirm this assumption. It was found that all C – C bonds in benzene have the same length (0.139 nm). They are somewhat shorter than single C – C bonds (0.154 nm) and longer than double (0.132 nm).
There are also compounds whose molecules contain several cyclic structures, for example:
Isomerism and the nomenclature of aromatic hydrocarbons
The homology of benzene is characterized by the isomerism of the position of several substituents. The simplest homolog of benzene – toluene (methylbenzene) – does not have such isomers, the following homolog is presented in the form of four isomers:
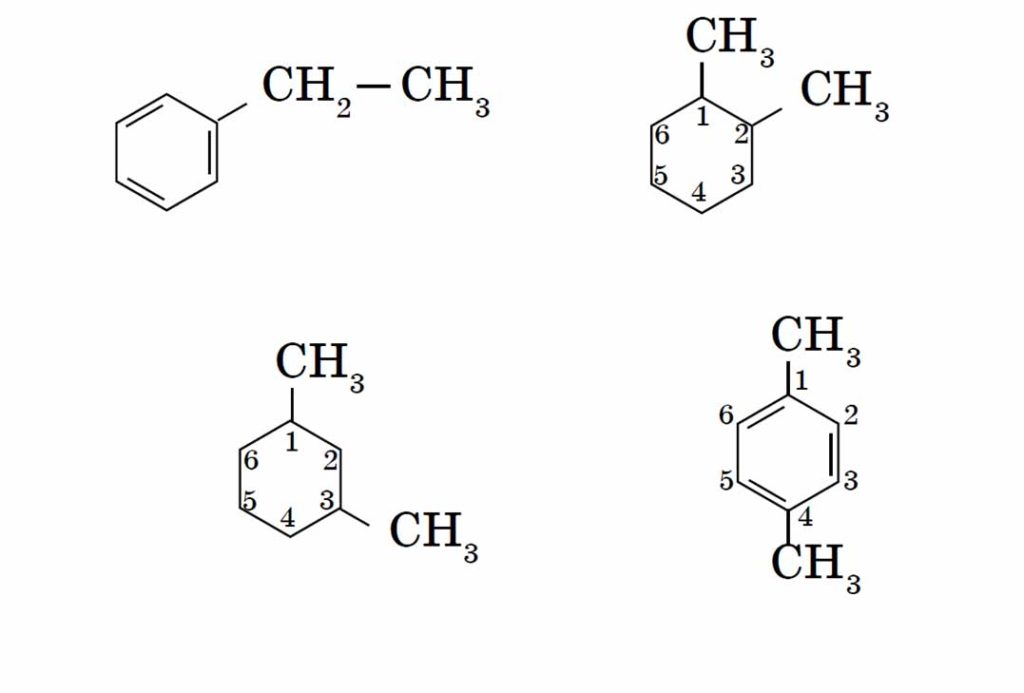
The name of the aromatic hydrocarbon with small substituents is based on the word benzene. The atoms in the aromatic ring are numbered, starting from the senior substituent to the youngest:

The American scientist L. Pauling proposed to represent benzene in the form of two boundary structures that differ in the distribution of electron density and are constantly passing into each other:
Structure and Stability
The hallmark of aromatic hydrocarbons is their cyclic, planar structure with conjugated pi electrons. This structure follows Hückel's rule, which states that a molecule is aromatic if it has pi electrons, where is a non-negative integer. For benzene, , giving it six pi electrons, which are delocalized over the carbon atoms, creating a stable electron cloud above and below the ring plane.

This delocalization of electrons imparts exceptional stability to aromatic compounds, often referred to as "aromatic stability." Benzene, for instance, is more stable than its hypothetical non-aromatic counterpart, 1,3,5-cyclohexatriene. This stability is reflected in benzene's reluctance to undergo addition reactions, which would disrupt its aromaticity.
Properties
Aromatic hydrocarbons exhibit several distinctive properties:
- Chemical Stability: As mentioned, the delocalized electrons confer significant stability, making these compounds less reactive than alkenes and alkynes.
- Physical Characteristics: Aromatic hydrocarbons are generally nonpolar and hydrophobic. They tend to have relatively high melting and boiling points due to the planar structure allowing tight packing in the solid state.
- Spectroscopic Features: Aromatic rings show characteristic absorption in UV-visible spectroscopy due to π-π* transitions. Additionally, aromatic protons appear in the 7-8 ppm range in proton NMR spectra.
Synthesis
Aromatic hydrocarbons can be synthesized through several methods:
- Electrophilic Aromatic Substitution (EAS): This is the most common method for introducing substituents onto an aromatic ring. Typical reactions include nitration, sulfonation, halogenation, Friedel-Crafts alkylation, and acylation.
- Cycloaddition Reactions: The Diels-Alder reaction, for example, can form six-membered aromatic rings by reacting a conjugated diene with a dienophile.
- Oxidative Coupling: Phenols can undergo oxidative coupling to form biphenyls and other polycyclic aromatics.
Reactions
Despite their stability, aromatic hydrocarbons do participate in a variety of chemical reactions, primarily through electrophilic aromatic substitution (EAS). In these reactions, an electrophile replaces a hydrogen atom on the aromatic ring. Some common EAS reactions include:
- Nitration: Introduction of a nitro group (NO₂) using nitric acid and sulfuric acid.
- Sulfonation: Addition of a sulfonic acid group (SO₃H) using sulfuric acid.
- Halogenation: Incorporation of halogens (Cl, Br) using halogen carriers like FeCl₃.
- Friedel-Crafts Alkylation and Acylation: Formation of alkyl or acyl groups using alkyl halides or acyl chlorides with AlCl₃ as a catalyst.
 Applications
ApplicationsAromatic hydrocarbons play a crucial role in various industrial and chemical processes:
- Solvents: Benzene, toluene, and xylene (BTX) are widely used as solvents in the chemical industry due to their ability to dissolve a broad range of substances.
- Polymers: Aromatic compounds are key monomers in the production of polymers like polystyrene and polyethylene terephthalate (PET).
- Pharmaceuticals: Many drugs contain aromatic rings, which contribute to their pharmacological activity. Examples include aspirin, paracetamol, and many antibiotics.
- Dyes and Pigments: Aromatic hydrocarbons are foundational in the synthesis of azo dyes and other colorants.
- Agricultural Chemicals: Herbicides, pesticides, and fungicides often contain aromatic structures that enhance their biological activity.
Environmental and Health Considerations
While aromatic hydrocarbons are invaluable in industry, they also pose environmental and health risks. Benzene, for instance, is a known carcinogen, and exposure can lead to serious health issues like leukemia. Therefore, handling and disposal of aromatic hydrocarbons require stringent safety protocols to minimize exposure and environmental impact.
In conclusion, aromatic hydrocarbons are a fundamental class of organic compounds with distinct structural features and stability. Their unique properties and versatility in chemical reactions make them indispensable in various industrial applications, ranging from solvent use to pharmaceuticals and polymers. However, their potential health risks necessitate careful management to ensure safety and environmental protection.
