Atomic Structure Chemistry || How do you find the Atomic Structure?
Atomic structure (also known as an atomic model) refers to the composition and arrangement of atoms and the arrangement and arrangement of parts.
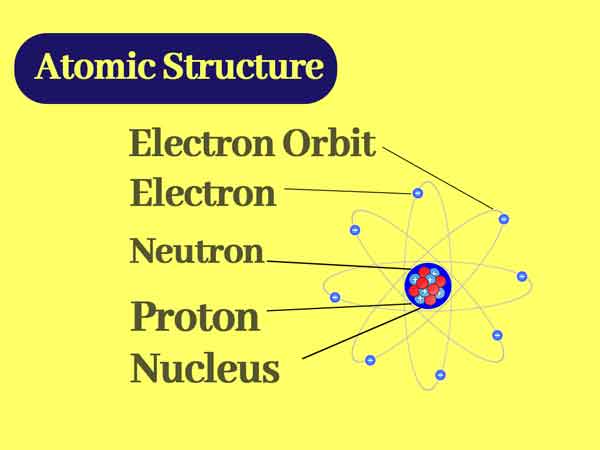
The atom is very small, taking carbon (C) as an example, its diameter is about 140pm (picometer), but it is usually recorded by the radius. In the case of a millimeter (mm), the diameter is 1.4 x 107mm, It consists of a nucleus located at the center of an atom and some tiny electrons.
These electrons move around the center of the nucleus, just like the planets of the solar system orbit the sun. And the atom is the same as any black particle in the universe. The latest research on the nucleus shows that the protons or neutrons in the nucleus may be spherical vibrational energy layers composed of two kinds of equilibrium forces, internal and external.
Using this principle, various kinds of relatively stable atomic nuclei can be constructed by using energy stack layers of different sizes. The entry details the neutral atom model, solid charged ball model, jujube cake model, Saturn model, solar system model, Ball model, nucleated model, and Chadwick model.
Discovery History
From the British chemist and physicist Dalton (J.John Dalton, 1766 ~ 1844) the creation of atomic theory after a long time people thought could not be more like a small atomic small glass medicine ball, which no longer No more tricks.
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
Since the discovery of cathode ray by German scientist Hitov in 1869, a large number of scientists such as Crouch, Hertz, Lerner, Thomson and others have studied cathode-ray for more than 20 years. Eventually, Joseph John Thomson discovered the existence of electrons.
Generally, atoms are uncharged. Since negatively charged electrons that are 1,700 times smaller in mass than atoms can be run from this atom, this indicates that there is a structure inside the atom and that there are still positively charged things in the atom. It should be neutralized with the negative charge carried by the electron to make the atom neutral.
Introduction of Atomic Structure
What else is there in the atom besides the Electron, how does the electron stay in the atom, what is the positive charge in the atom, how is the positive charge distributed, how does the negatively charged electron interact with the positively charged thing, etc. Wait for a whole bunch of new questions before physicists. Based on scientific practice and experimental observations at the time, physicists used their rich imagination to propose a variety of different atomic models.
1901 French physicist Perrin (Jean Baptiste Perrin, 1870-1942) structural model proposed that some of the central atoms are a positively charged particle, are some of the peripherals with the orbiting electrons, orbiting the period corresponding to the atom The frequency of the emitted spectral line, the outermost electrons are ejected to emit cathode rays.
Atomic model
Neutral atom model
In 1902 German physicist Lenard (Philipp Edward Anton Lenard, 1862-1947) proposed a neutral particle dynamic sub-model. Leonard’s early observations showed that cathode rays can pass through aluminum windows inside vacuum tubes and out of the tubes. Based on this observation, his absorption experiments in 1903 proved that high-speed cathode rays can pass thousands of atoms.
According to the prevailing semi-materialist view at the time, most of the volume of the atom was empty, and the rigid matter was only about 10-9 (or one hundred thousandth) of its total. Leonard imagined that “rigid matter” is a composite of several positive and negative electricity scattered in the internal space of the atom.
Solid Charged ball
The famous British physicist, inventor Kelvin (Lord Kelvin, 1824 ~ 1907), formerly known as W. Thomson (William Thomson), due to the installation of the first transatlantic submarine cable meritorious, the British government dubbed him a knight in 1866, and in Promoted to Lord Kelvin in 1892 and began to use the name, Kelvin. Kelvin has a wide range of research in thermal, electromagnetic, fluid mechanics.
He has contributed to optics, geophysics, mathematics, and engineering applications. He published more than 600 papers in his lifetime and obtained 70 patents for inventions. He enjoyed a high reputation in the scientific community at that time.
Kelvin proposed a solid charged sphere atom model in 1902, which regarded the atoms as a uniformly positively charged sphere with buried negatively charged electrons, which was in electrostatic equilibrium under normal conditions. This model was later developed by JJ Thomson, and later known as the Thomson atomic model.
Date cake model
Raisin cake model (jujube cake model)
Thomson (Joseph John Thomson, 1856-1940) continued his more systematic research in an attempt to delineate atomic structures. Thomson thought that the atom contained a uniform positron ball, and several negative electrons ran inside the sphere.
He followed Meyer on the floating (Alfred Mayer) magnet balance study demonstrated that if the number of electrons does not exceed a certain limit, these electrons run into a ring will be able to stabilize. If the number of electrons exceeds this limit, they will be listed in two rings, and so on.
In this way, the increase of electrons has caused periodic similarity in structure, and the repeated reproduction of physical and chemical properties in Mendeleev’s periodic table may also be explained.
In this model proposed by Thomson, the electron distribution in the sphere is a bit like raisins in a piece of cake. Many people call Thomson’s atomic model “raisin cake model “.
It can not only explain why the atoms are electrically neutral and how the electrons are distributed in the atoms, but also explain the phenomenon of cathode rays and the phenomenon that metals can emit electrons under the irradiation of ultraviolet rays.
And according to this model, the size of the atom can be estimated to be about 10 ^ -8 cm, which is a great thing. Because the Thomson model can explain many experimental facts at the time, it is easily accepted by many physicists.
Saturn model
Japanese physicist Hantaro Nagaoka (Nagaoka Hantaro, 1865-1950) 1903 December 5, in Tokyo mathematical physics oral presentation at the meeting, and were published in 1904 in Japanese, English, German magazine ” A paper describing the motion of electrons in atoms in linear and band-like spectra and radioactive phenomena.
He criticized Thomson’s model, arguing that positive and negative charges cannot penetrate each other, and proposed a structure he called a “Saturn model” -an atomic model that has an electron ring around a positively charged core.
A large-mass positively charged ball with a circle of equally spaced electrons on its periphery making circular motions at the same angular velocity. The electron’s radial vibration emission line spectrum, the vibration perpendicular to the torus surface emits the band spectrum.
The electrons flying out of the ring are beta rays, and the positively charged particles of the center sphere are alpha rays. This Saturnian model greatly influenced his later model of atomic nucleation. 1905 He [alpha] particle results of measurement of the charge mass ratio analysis, α particles are helium ions.
In 1908, Swiss scientist Leeds proposed a model of magnetic atoms.
Their model can explain some experimental facts of the time to a certain extent, but they cannot explain many new experimental results that appear later, so they have not been further developed. A few years later, Thomson’s “raisin cake model” was overthrown by his student Rutherford.
Solar system model
British physicist Ernest Rutherford (1871 ~ 1937) came to the Cavendish Laboratory in England in 1895 and studied with Thomson, becoming the first overseas graduate student of Thomson. Rutherford was diligent and diligent. Under Thomson’s guidance, Rutherford discovered alpha rays during his first experiment, the radiation absorption experiment.
Rutherford designed a clever experiment. He put radioactive elements such as uranium and radium in a lead container, leaving only a small hole in the lead container. Because lead can block radiation, only a small part of the rays are emitted from the small holes into a narrow beam of radiation.
Rutherford put a strong magnet near the radiation beam and found that a kind of ray was not affected by the magnet and kept traveling straight. The second type of ray is affected by the magnet and is deflected to one side, but it is not deflected much. The third type of rays is deflected extremely.
Rutherford put materials of different thicknesses in the direction of radiation and observed how the rays were absorbed. The first type of ray is not affected by the magnetic field, which means that it is uncharged and has strong penetrating power.
General materials such as paper and wood chips cannot block the progress of the ray. Only relatively thick lead The board can completely block it, called gamma rays. The second type of ray will be affected by the magnetic field to one side.
It can be judged from the direction of the magnetic field that the ray is positively charged. The penetrating power of this kind of ray is very weak, and it can be completely blocked by a piece of paper. This is the alpha ray discovered by Rutherford.
The third kind of ray is determined by the deflection direction to be negatively charged, and its properties are the same as those of fast-moving electrons, which are called beta rays. Rutherford was particularly interested in the alpha rays he found.
After in-depth and detailed research, he pointed out that alpha rays are a stream of positively charged particles. These particles are ions of helium atoms, that is, helium atoms missing two electrons.
The “counter tube” was invented by a student from Germany, Hans Geiger (1882-1945), and can be used to measure charged particles that are invisible to the naked eye. When the charged particles pass through the counting tube, the counting tube sends out an electrical signal. When this electrical signal is connected to the alarm, the instrument will make a “click” sound and the indicator light will be on.
Invisible rays can be recorded and measured with a very simple instrument. This instrument is called a Geiger counting tube. With the help of Geiger counting tubes, the research on the properties of alpha particles by the Manchester laboratory led by Rutherford has developed rapidly.
In 1910, E. Marsden (1889-1970) came to the University of Manchester, and Rutherford asked him to bombard gold foil with alpha particles, conduct practice experiments, and use fluorescent screens to record those alpha particles that passed through the gold foil.
According to Thomson’s raisin cake model, tiny mass electrons are distributed in a uniformly positively charged substance, while alpha particles are helium atoms that have lost two electrons, and their mass is thousands of times larger than electrons.
When such a heavy cannonball bombards the atom, the tiny electrons are irresistible. The positive matter in gold atoms is evenly distributed throughout the atomic volume, and it is impossible to resist the bombardment of alpha particles.
In other words, the alpha particles will easily pass through the gold foil, and even if they are blocked a little, it will only change the direction of the alpha particles slightly after passing through the gold foil.
Rutherford and Geiger have done this kind of experiment many times, and their observations are in good agreement with Thomson’s raisin cake model. The α particle is slightly changed in direction by the influence of gold atoms, and its scattering angle is extremely small.
Marsden and Geiger repeated this experiment that had been done many times, and a miracle appeared! They not only observed the scattered alpha particles, but also the alpha particles reflected by the gold foil.
In a speech in his later years in Rutherford, he described the scene at the time, saying: “I remember two or three days later, Geiger came to me with great excitement and said, ‘We got some alpha particles that reflected back.’, this is the most incredible event of my life.
This is as if you fired a 15-inch cannonball against the cigarette paper and was hit by the reflected cannonball. After thinking, I know this backscattering can only be the result of a single collision. After calculation, I can see that it is impossible to get this order of magnitude without considering that most of the atomic mass is concentrated in a small core. “
Rutherford’s “after thinking” is not thinking about one or two days, but thinking about a whole year or two. After doing a lot of experiments and theoretical calculations and deliberations, he boldly proposed a nucleated atom model, overthrowing his teacher Thomson’s solid charged ball atom model.
Rutherford examined the alpha particles reflected in his students’ experiments and carefully measured the total number of alpha particles reflected. Measurements show that under their experimental conditions, one alpha particle is reflected back for every eight thousand alpha particles incident.
Using Thomson’s solid charged sphere atom model and charged particle scattering theory can only explain small-angle scattering of alpha particles, but cannot explain large-angle scattering.
Multiple-scattering can obtain large-angle scattering, but the calculation results show that the probability of multiple-scattering is extremely small, which is far from the observation result that one of the eight thousand alpha particles reflected back.
The Thomson atom model cannot explain alpha particle scattering. Rutherford carefully calculated and compared it and found that only assuming that positive charges are concentrated in a small area, a large angle may occur when alpha particles pass through a single atom.
That is, the positive charge of the atom must be concentrated in a small core in the center of the atom. Based on this assumption, Rutherford further calculated some laws of α scattering and made some inferences. These inferences were quickly confirmed by a series of beautiful experiments by Geiger and Marsden.
The atom model proposed by Rutherford is like a solar system. Positively-charged nuclei are like the sun, and negatively-charged electrons are like planets orbiting the sun. In this “solar system”, the forces that govern them are electromagnetic interaction forces.
He explained that the positively charged matter in the atom is concentrated on a very small core, and most of the atomic mass is also concentrated on this very small core. When alpha particles are directed at the core of the atom, they may bounce back.
This satisfactorily explains the large-angle scattering of alpha particles. Rutherford published a well-known paper “Scattering and Principle Structure of Matter on Alpha and Beta Particles”.
Rutherford’s theory opened up a new way to study the structure of the atom, and made immortal contributions to the development of atomic science. However, for a long time at that time, Rutherford’s theory was coldly treated by physicists.
The fatal weakness of Rutherford’s atomic model is that the electric field force between positive and negative charges cannot meet the stability requirements, that is, it cannot explain how the electrons stay outside the nucleus stably. The Saturn model proposed by Changgang Bantairo in 1904 was unsuccessful because he could not overcome the difficulties of stability.
Therefore, when Rutherford put forward the model of nuclear atom, many scientists regarded it as a kind of conjecture or a variety of models, and ignored the solid basis on which Rutherford proposed the model. Experimental basis.
Rutherford has extraordinary insights, so he is often able to grasp the essence and make scientific predictions. At the same time, he has a very rigorous scientific attitude, and he draws conclusions that should be made based on experimental facts.
Rutherford believes that the model he proposed is still incomplete and needs further research and development. He stated at the beginning of the dissertation: “At this stage, it is not necessary to consider the stability of the atom mentioned, because obviously it will depend on the fine structure of the atom and the movement of the charged components.” “Hopefully, I’ll give some clearer insights into the atomic structure in one or two years.”
Bohr model
Rutherford’s theory has attracted a young man from Denmark, and his name is Niels Henrik David Bohr, 1885-1962), on the basis of the Rutherford model, He proposed the quantized orbit of electrons outside the nucleus, solved the problem of the stability of the atomic structure, and described a complete and convincing theory of atomic structure.
Bohr was born in a professor’s family in Copenhagen and received his PhD from the University of Copenhagen in 1911. He studied in Rutherford’s laboratory from March to July 1912, during which time he gave birth to his theory of atoms.
First, the Bohr Planck quantum of energy hypotheses extended to the inside of the atom, atomic model Rutherford to solve the difficulties in terms of stability, it is assumed only through the discrete atomic energy quantum changing its energy, i.e., only atoms It is in a discrete state, and the lowest state is the normal state of the atom.
Then he inspired the concept of steady-state transition from the combination law of spectral lines under the inspiration of his friend Hansen. He published a three-part paper “On Atomic and Molecular Structure” in July, September and November 1913.
Bohr ’s atomic theory gives such atomic images: electrons make circular motions around the nucleus in some specific possible orbits, and the farther away from the nucleus, the higher the energy, the angular momentum of the possible orbits must be an integer multiple of h/2π Decision, when an electron moves in these possible orbits, the atom does not emit or absorb energy, and the atom emits or absorbs energy only when the electron transitions from one orbit to another, and the radiation emitted or absorbed is single frequency, The relationship between radiation frequency and energy is given by E = hν. Bohr’s theory successfully illustrates the stability of atoms and the laws of the spectral lines of hydrogen atoms.
Bohr’s theory greatly expanded the influence of quantum theory and accelerated the development of quantum theory. In 1915, the German physicist Sommerfeld (Arnold Sommerfeld, 1868-1951) the Bohr atomic theory to include elliptical orbit, taking into account the mass of the electron effect of the special theory of relativity with varying speed, deriving the spectrum fine The structure is consistent with the experiment.
In 1916, Einstein (Albert Einstein, 1879-1955) analyzed the process of absorbing and emitting radiation from matter using statistical methods starting from Bohr’s atomic theory and derived Planck’s radiation law. This work of Einstein integrated the achievements of the first stage of quantum theory and combined the work of Planck, Einstein and Bohr into a whole.
Cored Model
Rutherford’s students have more than a dozen Nobel Prize winners, notably Bohr, Chadwick, Kocroft, Kapica, Hahn, etc. After the discovery of the nuclear, Rutherford in 1919 using alpha rays to bombard the nitrogen nucleus, for the first time in human history, “alchemy” was achieved, and for the first time, a nuclear reaction was achieved.
Since then, the element is no longer constant. Rutherford discovered that protons, that is, hydrogen ions, are the constituents of all atomic nuclei through a series of nuclear reactions, and predicted neutrons. Neutrons were later discovered by his student Chadwick, and the protons and neutrons were finally established as Basic nucleus structure model.
After the Pauli Exclusion Principle was established, the periodic law of elements was also explained. Rutherford was later known as the father of nuclear physics. Of course, just as the British were booming, don’t forget the French Curie couple, because Rutherford’s series of atomic shells needed for discovery were alpha particles emitted by radioactive elements (especially radium).
At this time, the Curie laboratory was established in France. Curie was killed in a car accident. Mary won the Nobel Prize for chemistry for her achievements in radioactivity. The famous book “General Theory of Radioactivity” was handed down.
Hosted by Joliot Curie and Elena Curie, they are equally talented and comparable to the three holy places. The little Curie couple had a bit of luck. They found that neutrons were preempted by Chadwick, positrons were preempted by Anderson, and nuclear fission was preempted by Hahn. The opportunity was fleeting.
But finally, because the discovery of artificial radioactivity won the Nobel Prize. Today, there are thousands of radioactive isotopes, most of which are artificially produced, thanks to the couple Curie.
The nucleated model succeeded experimentally, but there was a serious conflict with the basic theory at the time. According to classical electrodynamics, electromagnetic waves must be radiated due to the circular motion of electrons. Due to the loss of energy.
They will fall into the nucleus within 1ns and emit a continuous spectrum at the same time. In other words, there is no such thing as an atom in theory. However, atoms do exist and are stable. They emit linear spectra, which are supported by a large number of experimental facts and the entire chemistry.
In 1911, a 26-year-old Danish young man came to Cambridge and then transferred to the Rutherford Laboratory in Manchester to learn about the amazing discovery of the nucleus. In the end, he found a fundamental modification of the nucleation model, which can both illustrate the stability of the atom and calculate the radius of the atom. He was Niels Bohr, who was on par with Einstein.
In 1885, a mathematics teacher in Switzerland, Balmer, discovered an empirical formula for the visible spectrum of hydrogen atoms, which was later generalized by the Swedish physicist Derberg to the Rydberg formula.
In 1900, the German physicist Planck proposed the concept of quantization of energy and explained the black-body radiation spectrum. In 1905, Einstein proposed the concept of light quantum. These conclusions greatly inspired Bohr. Under these revelations, Bohr applied the concept of quantization to the atom model in 1913 and proposed Bohr’s hydrogen atom model.
The key to this model is the three assumptions introduced by Bohr. Stationary assumption: electrons can only move in some discrete orbits, and they will not radiate electromagnetic waves.
Frequency condition assumed level difference between the atomic absorption (or emission) of photon same energy. The quantization of angular momentum assumes that the angular momentum of the electron is an integer multiple of the flower Planck constant.
Through a series of derivations, the mystery of the hydrogen spectrum gradually surfaced with great success. Bohr won the Nobel Prize in 1922. Although the Bohr model now seems rough, its significance is not in the model itself, but in the concepts introduced when the model was established: steady-state, energy levels, transitions, etc. Bohr introduced the correspondence principle, coordinated the conflict between the hydrogen atom model and classical mechanics.
After Bohr’s success, he rejected the invitation of the mentor Rutherford, returned to the motherland, and established an institute in Copenhagen (later renamed the Bohr Institute). The Bohr Institute attracted a large number of outstanding young physics from around the world Scientists, including Heisenberg, Pauli, and Dirac, the founders of quantum theory, formed a strong academic atmosphere. At this time, Copenhagen began to explore the basic laws of physics.
Until now, physics can be roughly divided into two schools. One is the classical school of physics represented by Einstein, and the members are roughly Planck, DeBroy, Schrödinger, etc. The other is Copenhagen School led by Bohr The members are roughly Bonn, Heisenberg, Pauli, Dirac and so on. Naturally, this debate has not yet come to fruition. So what happened to physics after the Bohr hydrogen atom? What is the point of contention between the two scientific giants?
Chadwick Model
In 1935, British physicist Sir James Chadwick was born in England in 1891. After graduating from the University of Manchester, he specialized in the study of radioactive phenomena. Later, he went to Cambridge University and achieved many results under the guidance of Professor Rutherford. In 1935, he won the Nobel Prize in Physics for discovering neutrons. During World War II, he went to the United States to conduct nuclear weapons research. He died in 1974.
He found that neutrons and protons had the same mass, but he was uncharged. The existence of neutrons explains why the mass of atoms is greater than the total mass of protons and electrons. He also won the 1935 Nobel Prize for discovering neutrons.
An atom is made up of a positively charged nucleus and a negatively charged electron orbiting the nucleus. The mass of the atom is concentrated almost entirely on the nucleus. At first, it was thought that the mass of the nucleus (according to Rutherford and Bohr’s atomic model theory) should be equal to the number of positively charged protons it contains.
However, some scientists have found in research that the number of
positive charges of an atomic nucleus is not equal to its mass! That is,
in addition to the positively charged protons, the nucleus should also
contain other particles. So what is that “other particle”?
To
solve this physical problem and discover that the “other particles” are
“neutrons” is the famous British physicist James Chadwick.
In 1930, when scientists Bott and Baker bombarded beryllium with alpha particles, they found a very penetrating ray.
They thought it was gamma rays and ignored them. Webster even
carefully identified this radiation and saw its neutral nature, but it
was difficult to explain this phenomenon, so he did not continue to
study it further. Madame Curie’s daughter, Elena Curie, and her husband
also wandered on the brink of “Beryllium Rays” and ended up missing
neutrons.
Chadwick was born in Cheshire, England in 1891 and graduated from Victoria University of Manchester. No talent was shown in middle school. He is quiet and mediocre, but insists on his credo: If you can do it, you must do it right, and you are meticulous; if you do n’t do it, you do n’t understand it.
Therefore, he sometimes fails to complete the physical work on schedule. It is precisely this spirit of vanity, seeking truth from facts, and “doing ten things in one’s own way that makes one’s work hard”, which has benefited him in his scientific research career.
Chadwick, who entered the university, quickly showed his talent in physics research because of the solid foundation of knowledge. He was fascinated by the famous scientist Rutherford. After graduation, he stayed in the Physics Laboratory of the University of Manchester and engaged in radiological research under the guidance of Rutherford.
Two years later, he was awarded a British National Scholarship for
his successful experiment of “the alpha rays deviate through the metal
foil”.
At the dawn of his scientific research career, World War I
put him in a civilian prisoner camp, and it was not until the end of the
war that he was free and returned to scientific research.
In 1923, he was promoted to the Deputy Director of Cavendish
Laboratory of Cambridge University for outstanding results in the
measurement and research of nuclear charge and worked with director
Rutherford on particle research.
In 1931, Mrs. and Mrs. Curio’s
daughter and son-in-law announced their new discovery about a large
number of protons produced by paraffin wax under the irradiation of
“beryllium rays”. Chadwick immediately realized that the rays were
likely composed of neutral particles, which were the key to solving the
mystery that the positive charge of the nucleus was not equal to its
mass!
Chadwick immediately set out to study an experiment
performed by the Joliot Curie couple, using a cloud chamber to measure
the mass of such particles and found that the particles had the same
mass as protons and had no charge. He called such particles “neutrons.”
The neutron was discovered by him. He solved the problems encountered
by theoretical physicists in atomic research and completed a
breakthrough in atomic physics research. Later, Italian physicist Fermi
bombarded the uranium nucleus with neutrons as “cannonballs”, discovered
nuclear fission and chain reactions in fission, and opened a new era of
human use of atomic energy. Chadwick was awarded the Nobel Prize in
Physics in 1935 for his outstanding contribution to the discovery of
neutrons.
Quantitative relationship
Quantitative relationship between the structural particles that make up an atom
① Mass number (A) = number of protons (Z) + number of neutrons (N)
② Number of protons = number of nuclear charges = number of electrons outside the nucleus = atomic number
Note: The neutron determines the type of atom (isotope), the mass number determines the approximate relative atomic mass of the atom, the number of protons (nuclear charge number) determines the type of element, the number of electrons in the outermost layer of the atom determines the apparent or non-electrical nature of the atom, and it also determines Chemical properties of main group elements.