Atomic Weight and Mass Number: Mass Spectrometer and amu
The atomic weight of an element is a number that shows how many times
the weight of one atom of that element is 1/12 heavier than that of an
atom of carbon-12. Therefore, it is clear from the above definition that
–
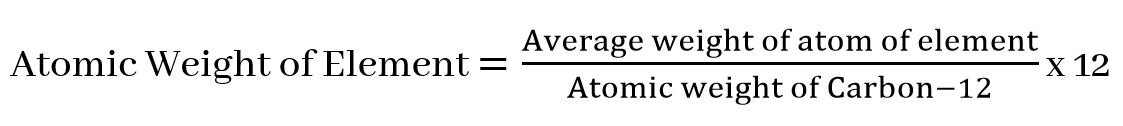
From the above definition it is clear that:-
1. Atomic weight is a number and has no units.
2. Atomic weight of carbon-12 = 12
3. The mass of carbon is 12. Hence, the atomic mass and carbon mass of carbon-12 are the same. Similarly, it can be demonstrated that the atomic weights of other elements are also approximately equal to their mass numbers. Therefore
Atomic weight = Mass Number
Isotopes have different mass numbers. Hence their atomic weights are also different. The atomic weights of isotopes are also called Isotopic Masses.
Hydrogen has three isotopes 1H1 1H2 and 1H3 known. An atom of 1H1 is 1.007825 times heavier than 1/12 of an atom of carbon-12, hence the atomic weight of 1H1 is 1.007825.
Similarly, atomic weight of 1H2 is 2.0140 and atomic weight of 1H3 is 3.01605. In other words the isotopic masses of 1H1 1H2 and 1H3 are 1.007825 2.0140 and 3.01605 respectively.
In the above definition, the use of the term ‘average’ shows that all atoms of any element have the same mass.
So far 112 elements have been discovered. Isotopes of all elements are known. Different isotopes of a single element have different mass and their atomic weights.
Therefore, to find the atomic weight of an element, the average weight of all the atoms of that element must be known.
Example:
The atomic functions of nitrogen-14 and nitrogen-15 found in nature are in the ratio of 99.63 and 0.37%.
The atomic weight of nitrogen-14 is 14.00307, which means that one atom of nitrogen-14 is 1/12 to 14.00307 times heavier than one atom of carbon-12.
Similarly, the atomic weight of nitrogen-15 is 15.00011 which means that one atom of nitrogen-15 is 15.00011 times heavier than 1/12 of one atom of carbon-12.
Atomic Weight of nitrogen-14 = 14.00307
Atomic Weight of nitrogen-15 = 15.00011

Carbon found in nature, the ratio of carbon isotopes carbon-12 and carbon-13 is 98.89% and 1.11%.
According to the definition of atomic weight, the atomic weight of carbon-12 is 12. The weight of one atom of carbon-13 is 13.00335 times that of 1/12 of an atomic weight of carbon-12.
Atomic Weight of Carbon-12 = 12
Atomic Weight of Carbon-13 = 13.00335
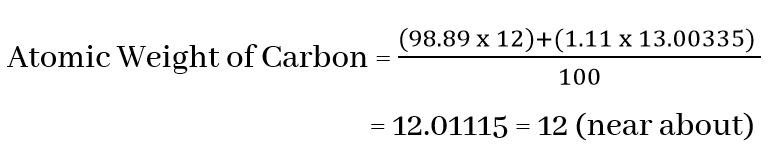
To find the atomic weight of an element, the average weight of 1 atom of that element is compared to 1/12 of the weight of one atom of carbon-12. It should be noted that it is comparable to the isotopic mass of 12 mass numbers of carbon (Carbon-12) atom and not natural carbon atom (mixture of Carbon-12 and carbon-13).
In this definition, it is assumed that the atomic weight of carbon-12 is 12. In the old definition of atomic weight it was compared to an atom of hydrogen.
According to that definition, the atomic weight of hydrogen was assumed to be 1.
By that definition, the atomic weights of many elements differed significantly from the whole number. According to the current and modified definitions, the atomic mass of elements is relatively close to absolute numbers.
Hence, the definition of atomic mass in which the average weight of one atom of an element was compared to the weight of one atom of hydrogen has been changed and according to the current modified definition, the weight of one atom of carbon-12 is 1/12. The atomic weight of hydrogen is 1.008 according to the current modified definition.
Concentration of the Ore
According to the definition of atomic weight, in order to find the atomic weight of an element, the ratio of the weight of one atom of that element to that of an atom of carbon-12 must be found.
This ratio is determined with the help of mass spectrometer. The mass spectrometer was first built by Aston 1919. The brief description of mass Spectrometer is as follows.
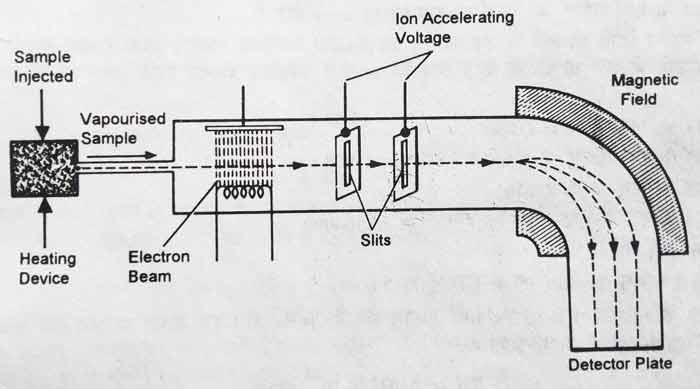
The element whose atomic weight has to be determined is taken in gaseous state. In the gaseous state, that element flows through a higher electric field.
The electrons undergo ionization and their cations are obtained by hitting the atoms of the gas. These cations pass through such an electric field that their speed is accelerated, after which they pass through a magnetic field.
Under the influence of the magnetic field, there is a deflection in their path. The lighter the cation, the greater the deflection in its path. After the deflection, the hypotenuse collides with a photographic plate.
On the photographic plate, based on the signs obtained from different curtains, their therapeutic ratio can be determined. On the basis of the sizes of the signs, the natural abundance of the institutes of an element can also be found.
Example
Bellow results are obtained by analysing a sample of natural neon with a modern mass spectrometer.
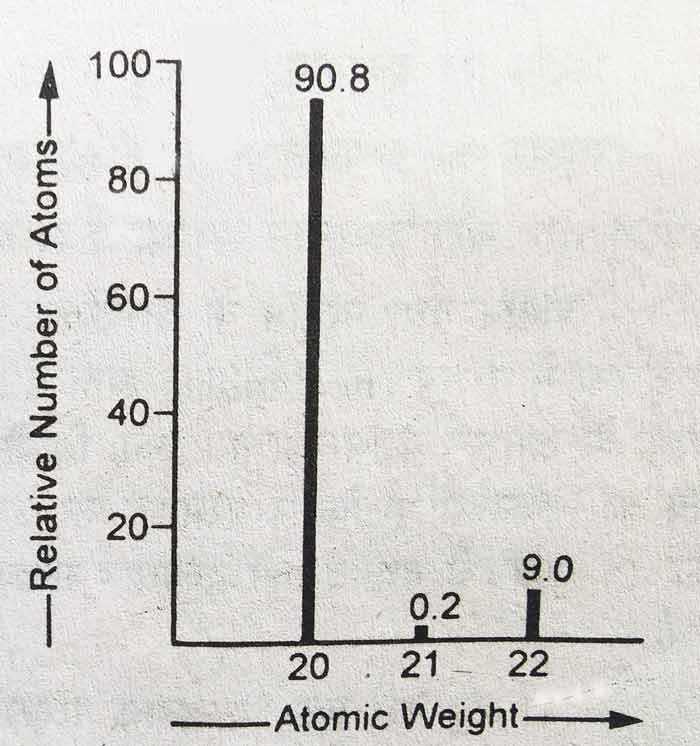
Gram-atomic Weight
If the atomic mass of an element is expressed in grams, it is called the gram-atomic mass of that element.
Example
The atomic weight of nitrogen is 14.00676, so 14.00676 grams is the gram-atomic weight of nitrogen.
Atomic Mass unit(amu)
The unit of mass is gram. It is not convenient to dissolve the actual mass of small particles like electron protons, neutrons, atoms, molecules and ions into grams. “Gram” is a large unit for these small particles.
So in 1961, a suitable set up to show the actual mass of these particles was established. This unit is called atomic mass unit(amu).
An atom of carbon-12 has an actual mass of 12 amu.
On this basis, the actual mass in the amu of an atom of any element is equal to its atomic weight. Similarly, the actual mass in the amu of 1 molecule of any element or additive is equal to its molecular weight.
Example:
Atomic weight of Hydrogen = 1.008
Actual mass of one atom of hydrogen = 1.008 amu
Molecular weight of hydrogen = 2.016
Actual mass of one molecule of hydrogen = 2 .016 amu
Molecular weight of HCI = 36 .5
Actual mass of one molecule of HCI = 36 .5 amu
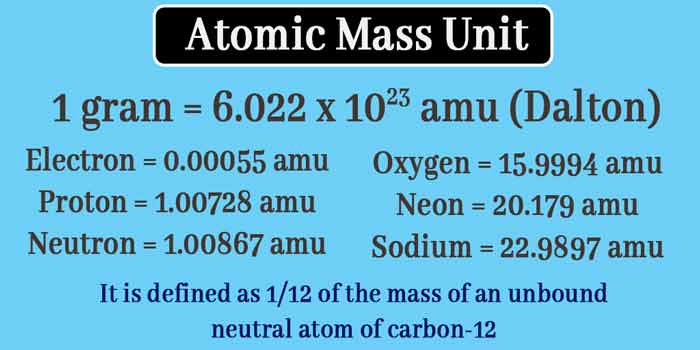
There are 6.022 x 1023 atoms in 12 grams of carbon-12, on this basis the relationship between gram and amu is known.
1 gram = 6.022 x 1023 amu
When the relationship between gram and amu is known, the masses of the original particles of the curtain can be expressed in both units.
Mass of Electron = 9.1095 x 10-28 gram = 0.00055 amu
Mass of Proton = 1.6726 x 10-24 gram = 1.00728 amu
Mass of neutron = 1.6750 x 10-24 gram = 1.00867 amu
Atomic Mass and Mass Number
The actual masses of protons and neutrons are about 1 amu. The mass of the electron is negligible compared to the mass of these particles.
Hence, the actual mass in the amu of an atom of an element will be approximately equal to its mass number.
Hence, the atomic weight of an element is approximately equal to its mass number.
The atomic weight of an element in its nearest integer is equal to its mass number.
Example: Atomic weight of oxygen-16 is 15.99949 and its mass number is 16. The atomic weight of hydrogen-1 is 1.007825 and its mass number is 1.
The atomic weight is a fractional number – the atomic weight of most elements is close to a whole number, however the atomic weight is a fractional number, for the following reason –
1) The mass in the amu of the electron, proton, and neutron is fractional. The actual mass of an atom should be equal to the sum of the masses of electrons, protons, and neutrons present in it. Therefore, the actual mass of an atom in amu is fractional. Since the actual mass in the amu of an atom is equal to its atomic mass, the atomic mass is a fractional number.
2) Due to the mass loss of the nucleus, the actual mass of an atom is slightly less than the sum of the mass of electron protons and neutrons present in it, so the actual mass in the amu of the atom will be fractional. Hence the atomic weight is a fractional number.
3) The atomic weights of elements are related to the average mass of all their isotopes. Since the average mass of the elements of the elements is fractional. Hence, atomic mass is a fractional number.
