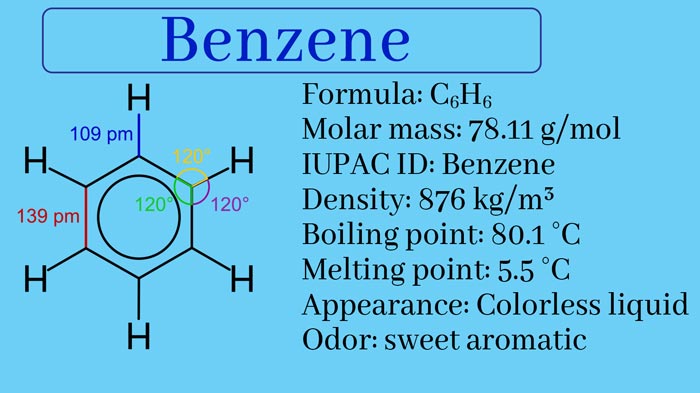
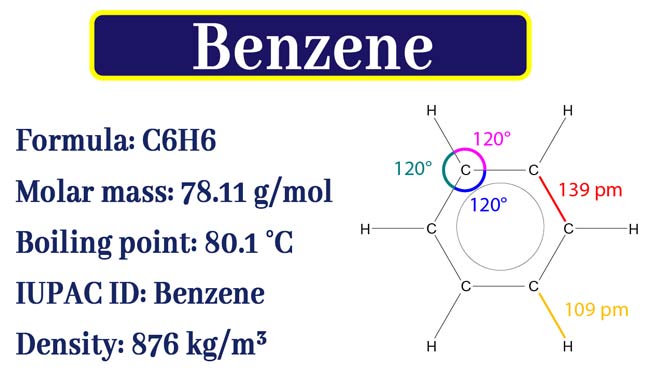
What do you mean by Benzene? Preparation and Properties
Benzene was first discovered by Michael Faraday in 1825. Faraday obtained benzene from a distillation of while fish oil. Hofmann obtained it in 1845 from the fractional distillation of bitumen. A few decades from now, bitumen(coil tar) was the major source of benzene. Now the major source of benzene is petroleum.
Benzene is the basic compound of the aromatic group. Most aromatic compounds are derivatives of benzene. All aromatic compounds have a cyclic structure like benzene or Toluene.
Benzene preparation
Laboratory method: sodium benzoate
In the laboratory, benzene is made by distillation of sodium benzoate and soda lime.
C6H5COONa + NaOH → C6H6 + Na2CO3
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
A round bottom distillation flask takes about 30 grams of sodium benzoate and about 45 grams of soda lime. Gently heat the condenser by placing it in the flask.
Benzene is collected as distilled with water. The benzene being insoluble in water forms a separate surface with water, which is separated by a practical funnel. Thus obtained benzene is dried on anhydrous calcium chloride to obtain pure benzene by re-distillation.
Acetylene
Berthelot synthesized benzene from acetylene in 1870 when acetylene is transported into the red heated tube and benzene is obtained.
3C2H2 → C6H6
Chlorobenzene
Benzene is obtained by reduction of Chlorobenzene by Ni – Al alloy and NaOH.
C6H5Cl + 2H → C6H6 + HCl
Phenol
Benzene is obtained by heating Phenol with zinc powder.
C6H5OH + Zn → C6H6 + ZnO
Benzenesulfonic Acid
Benzene is obtained by boiling Benzenesulfonic acid with dilute HCl or dilute H2SO4, or when heated with steam.
C6H5SO3H + H2O → C6H6 + H2SO4
Aniline
Benzenediazonium chloride is obtained when the aniline is reacted with a mixture of NaNO2 and HCl at 0-5°C. Benzene is obtained by reduction of Benzenediazonium chloride with hypophosphorous acid(H3PO2), formic acid or ethanol, Stanous chloride.
C6H5NH2 + HNO2 + HCl → C6H5-N=N-Cl + 2H2O
C6H5-N=N-Cl + 2H → C6H6 + N2 + HCl
Coal tar
It is obtained from the efficient distillation of Coal tar in commercial quantities.
When distillation of Coal tar at 170°C a fractional liquid obtained. It is called light oil. It is lighter than water.
In addition to benzene, toluene and xylene, basic substances like pyridine and acidic substances like phenol and cresol are present. To remove them, light oil is first reacted with sodium hydroxide, which removes acidic impurities. After this, its diluents are reacted with H2SO4, which results in the separation of basic substances such as pyridine.
Now the light oil is washed with water. Due to which acidic elements are removed. In this way, efficient distillation of purified oil is done, which gives the following effect.
- Gauss Law or Gauss Theorem : Solid Angle and Electric Flux
- Electrolysis : Definition, Principle and Electrolytic Cell
- Adsorption : Adsorption rate, Physisorption and Chemisorption
- Electric Cell : E.M.F., Terminal Potential, Internal Resistance
- How Transistor Works : PNP and NPN Transistors
- How p-n Junction Diode works : Forward and Reverse Biasing
- Semiconductors : How Semiconductor works and Types
- X-Rays – Production, Properties, Wavelength and Uses
90% benzoyl (80 – 110°C) – 90% benzoyl means that distillation of 100 ml at 100°C gives 90 ml distillate. It contains 70% benzene and the rest toluene and xylene.
50% benzoyl (110 – 140°C): 50% benzoyl means that distillation of 100 ml at 100°C gives 50 ml distillate, it contains 45% benzene and the remaining toluene, xylene and other hydrocarbons.
Solvent naphtha or benzoic 140 – 170°C – It is used in dissolving dyes, rubber and resins, on re-effecting distillation of the above constituents, benzene at 80°C gives toluene at 110°C and xylene at 135°C.
Petroleum
In commercial quantities it is obtained from aromatization of n-heptane obtained from petroleum.
It’s C6 – C8 component is heated at high pressure and high temperature in the presence of appropriate catalysts to form benzene, toluene and xylene from petroleum.
In this action, benzene toluene and xylene are obtained as a result of aromatisation of aliphatic and alicyclic hydrocarbons. This action is called catalytic reforming or plat forming. Its ingredients are separated by Fractional distillation from a mixture of benzene, toluene and xylene.
Benzene Physical Properties
Benzene is a colourless, volatile, flammable and distinctly smelling liquid. Its boiling point is 80.4°C and freezing point is 5.4°C. It is insoluble in water and due to being lighter than water, forms a separate surface over the water.
It is soluble in alcohol, ether and chloroform. It is a good solvent for resins, fats, sulphur and iodine.
Benzene Chemical Properties
The chemical reactions of benzene can be divided into three parts.
- Addition reactions
- Oxidation reactions
- Substitution reactions
Addition of Hydrogen
Cyclohexane is obtained as a result of high pressure in the presence of nickel or platinum and the addition of benzene and hydrogen at about 250°C.

Addition of Halogens
Benzene Hexachloride is obtained as a result of the reaction of chlorine with benzene in the presence of sunlight (hv).
C6H6 + 3Cl2 → C6H6Cl6
Banzene Hexachloride is also known as BHC, gammaxene, Lindane or 666. It is a strong germicide. The addition of benzene to bromine is similar.
Oxidation Reactions
Ozonolysis
The addition of benzene to the ozone results in the addition of benzene triozonide, which results in three atoms of Glyoxal by water decomposition.

The addition of benzene to the ozone results in the addition of benzene triozonide, which results in three atoms of Glyoxal by water decomposition.
Catalytic Oxidation
By heating at 450°C in the presence of vanadium pentoxide(V2O5), it is oxidized by oxygen in the air to form a maleic anhydride.

Substitution Reactions
Halogenation
Benzene reacts with chlorine or bromine at room temperature in the absence of sunlight and in the presence of halogen carriers such as Fe or FeCl3 to form replacement products.
C6H6 + Cl2 → C6H5Cl + HCl
C6H6 + Br2 → C6H5Br + HBr
Nitration
Benzene reacts with concentrated nitric acid in the presence of concentrated sulfuric acid to form nitrobenzene.
C6H6 + HNO3(conc.) → C6H5NO2 + H2O
This reaction occurs at a slower rate than normal temperature and faster at increasing temperature. At higher temperatures and greater use of nitric acid, di and tri substitution products ie m-dinitro benzene and 1,3,5 tri-nitro-benzene are obtained.
Sulphonation
benzene sulphuric acid is obtained by heating benzene with concentrated sulphuric acid. This reaction takes place with ordinary sulphuric in normal temperature.
C6H6 + H2SO4 → C6H5SO3H + H2O
Friedel-Crafts alkylation
The reaction of benzene to an alkyl halide in the presence of a lewis acid such as AlCl3 results in alkylation of benzene.
C6H6 + CH3Cl → C6H5CH3 + HCl
Friedel-Crafts Acylation
The reaction of benzene to an acyl halide in the presence of a lewis acid such as AlCl3 results in acylation of benzene.
C6H6 + CH3COCl → C6H5COCH3 + HCl
Gattermann Koch Aldehyde Synthesis
Benzaldehyde is obtained when a mixture of carbon mono oxide and hydrogen chloride gas flows into benzene in the presence of AlCl3. This reaction is called Gattermann Koch Aldehyde Synthesis.
C6H6 + CO → C6H5CHO
Gattermann Aldehyde Synthesis
Benzaldehyde is obtained when a mixture of carbon mono oxide and hydrogen chloride gas flows into benzene in the presence of AlCl3. This reaction is called Gattermann Koch Aldehyde Synthesis.
C6H6 + HCN → C6H5CH=NH + HCl
C6H5CH=NH + H2O → C6H5CHO + NH3
Chloromethylation
benzene chloride is obtained when a mixture of pharmaldehide and HCl gas flows into benzene in the presence of anhydrous ZnCl2. In this reaction, a hydrogen atom present in the benzene ring is displaced by a chloromethyl (-CH2Cl)group.
C6H6 + CH2O + HCl → C6H5CH2Cl + H2O
Mercuration
In alcoholic solution, benzene is heated with mercuric acetate to obtain Phenylmercuric acetate.
C6H6 + (CH3COO)2Hg → C6H5-Hg-O-CO-CH3 + CH3COOH
Benzene uses
It is used to make many other aromatic compounds. Many aromatic compounds are used as dyes, medicines, explosives, and other useful substances.
It is also used in dry cleaning of clothes.
A mixture of benzene and petrol is used as fuel in motor cars.