Benzoic Acid: Formula, Structure, Properties, Uses, and Tests
Preparation of Benzoic Acid
Laboratory Method
Benzyl chloride: Benzoic acid is obtained by oxidation of benzyl chloride by alkaline KMnO4.
2KMnO4 + 2KOH → 2K2MnO4 + H2O + O
C6H5CH2Cl + KOH → C6H5CH2OH + KCl
C6H5CH2OH → C6H5CHO → C6H5COOH
Using KOH in the above reaction, potassium salt of benzoic acid is obtained at the end of the reaction. Once it is acidified by SO2 gas, benzoic acid is released.
Method used
To make Benzoic Acid in the laboratory, take 5 gram benzyl chloride, 4 gram sodium carbonate, 8 gram KMnO4 and about 200 ml water in a round flask. Apply a reflux condenser to the flask and heat it until the pink color of KMnO4 fades.
After cooling the flask mixture to about 80°C, the SO2 gases flow in it until the precipitate of MnO2 completely dissolves. Crystals of Benzoic Acid dissociate upon cooling the mixture. After filtering them, washing them and drying them, pure benzoic acid is obtained.
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- What is Urea || How to make Urea Fertilizer, || Urea uses
Benzyl alcohol, benzaldehyde or Toluene: benzoic acid is obtained by oxidizing any of these three compounds with acidic or alkaline KMnO4 or acidic K2Cr2O7.
C6H5CH3 → C6H5CH2OH → C6H5CHO → C6H5COOH
Phenyl cyanide: Phenyl cyanide is also called cyanobenzene or benzonitrile. Benzoic acid is obtained from its acid water decomposition.
C6H5CN + 2H2O → C6H5COOH + NH3
Benzoyl Chloride: Benzoic acid is obtained by reaction of benzoil chloride with water.
C6H5COCl + H2O → C6H5COOH + HCl
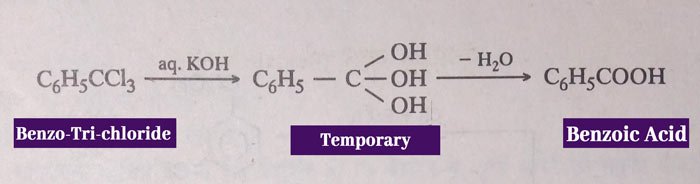
Benzotrichloride: benzoic acid is obtained by reaction of benzotrichloride with aqueous NaOH or KOH.
Physical Properties
Benzoic acid is a white solid crystal. Its melting point is 122°C. It is less soluble in cold water but more soluble in hot water, ethanol and ether. Its vapour irritates the nose and throat.
Chemical Properties
Acidic character: It reacts with bases to form salts. Example:
C6H5COOH + NaOH → C6H5COONa + H2O
C6H5COOH + NaHCO3 → C6H5COONa + H2O + CO2
Benzoic acid is a stronger acid than acetic acid. The reason for this is that the carboxyl group in benzoic acid is associated with the minus-denatured phenyl group. Hence the amount of partial positive charge on H of carboxyl group in benzoic acid is high.
The carboxyl group in acetic acid is associated with the methyl-methyl group. Therefore, the amount of partial positive charge on H of carboxyl group in acetyl acid is less. Hence benzoic acid is a stronger acid than acetic acid.
- Benzoic Acid: Formula, Structure, Properties, Uses, and Tests
- Benzaldehyde Formula, Preparation, Properties, uses, and Tests
- What is phenol used for? Preparation, Properties, uses, and Tests
- What is Benzenesulfonic Acid used for? Preparation, Properties, and uses
- What is aniline used for? Preparation, Properties, and Tests
- How do you make Nitrobenzene? | Properties, Tests, and uses
- What is the formula of Chlorobenzene? Preparation, Properties and uses
- NEET 2021 / JEE 2021 Crash Course for Chemistry Subject
Esterification: Like other acids, benzoic acid also reacts with alcohols to form esters. Example:
C6H5COOH + C2H5OH → C6H5COOC2H5 + H2O
Decarboxylation: Decarboxylation occurs when benzoic acid is heated with soda lime like other carboxylic acids.
C6H5COOH + NaOH → C6H5COONa + H2O
C6H5COONa + NaOH → C6H6 + Na2CO3
Reaction with phosphorus pentachloride: Like other carboxylic acids, benzoic acid reacts with PCl5 to form acid chloride.
C6H5COOH + PCl5 → C6H5COCl + POCl3 + HCl
Reducation: It is reduced by H2 / Ni, Na / Alcohol, LiAlH4 or NaBH4 to form benzyl alcohol.
C6H5COOH + 4H → C6H5CH2OH + H2O
Replacement reactions of nuclei: The carboxylic group is a meta directing group. Hence meta products are derived from its helogenation, nitration, sulphonisation.

Benzoic Acid uses
It is used as a medicine and in the manufacture of medicines.
It is used in the manufacture of dyes called aniline blue.
sodiuam benzoate is used in preservation of many foods like fruits, pickles, tomato sauce, fruit juice, etc.
Benzoic Acid Tests
This gives a normal NaHCO3 test of carboxylic acid. Therefore, on adding a saturated solution of NaHCO3 to benzoic acid, CO2 gas exits with intense bubbling.
Specific tests are as follows.
Addition of ferric chloride(FeCl3) to a neutral solution of benzoic acid results in a camel hair-like buff precipitate.
Flammable vapor of benzene is obtained when benzoic acid is heated with soda lime.