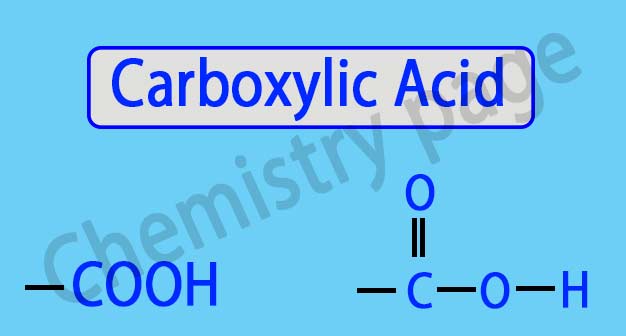
Carboxylic Acid: What is the common name of a carboxylic acid?
Carboxylic Acid: The –COOH group is called a Carboxylic Group. Compounds that contain one or more carboxylic groups are called Carboxylic Acids. Depending on the number of carboxylic groups present in carboxylic acids, they are called mono carboxylic, di-carboxylic or tri-carboxylic acid.
Example:

Mono carboxylic acids in which the -COOH group is attached to a hydrogen atom or an alkane group are called alkanoic acids. Their common formula is CnH2nO2 or RCOOH, where R = H or alkane group.
They can be arranged as a homogeneous series. Higher members of this series such as Palmitic acid (C16H32O2) and Stearic acid (C₁₇H₃₅CO₂H) are found in fats. Hence, members of this series are also called Fatty Acids.
Nomenclature of Carboxylic Acid
The common names of the first four members of this series are based on their sources. One source of HCOOH is Red Ant. Hence its common name is formic acid. One source of CH3COOH is vinegar. Hence its common name is acetic acid.
To name the members of this series in the IUPAC method, we add ‘oic acid’ to the end of the corresponding alkane name. Remove ‘e’ from the end of English name and add ‘oic acid’.
Since the -COOH group in these compounds is always present at one end in the chain of carbon atoms, the number of carbon atoms belonging to the -COOH group in the chain of carbon atoms in these compounds is always 1.
Therefore, it is not necessary to display this number when writing the IUPAC names of these compounds.
Following are the common and IUPAC names of some carboxylic acids.
Carboxylic Acid Isomerisms
Carboxylic acid with similar molecule formula exhibits interconnected chain isomerism. These compounds have differences in the structures of chains of carbon atoms. Example: n – butyric acid and iso butyric acid are interchangeable series.
Carboxylic acid and carboxylic ester having the same molecule formula exhibit interactive isomerism. The functional groups present in these compounds differ.
Carboxylic Acid #1| Introduction, Nomenclature
The following points are discussed here
1. Introduction carbonyl Compounds
2. Nomenclature carbonyl Compounds
3. Structure of carbonyl Compoundvs
<iframe width="834" height="469" src="https://www.youtube.com/embed/mJxqEqXDp3Y" title="Aldehyde, Ketones and Carboxylic Acid #1| Introduction, Nomenclature, Structure of Carbonyl Compound" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share" referrerpolicy="strict-origin-when-cross-origin" allowfullscreen></iframe>
Example: The molecule formula C3H6O2 represents one carboxylic acid and one carboxylic ester. These two compounds are functional isomerism of each other.
Propionic acid: CH3CH2COOH
Methyl Acetate: CH3COOCH3
Relative Strengths of Carboxylic Acids
Carboxylic acid exhibits acidic properties and reacts with alkalis to make salt and water.

Each carboxylic group is capable of delivering one protein. Therefore, the basicity of mono carboxylic acids is 1 and they are also called monobasic acids.
The reason for the acidic properties of carboxylic acids is that the carbonyl group is polar. For this reason, a positive charge is present on the carbon atom of the carbonyl group. Due to this, partial positive charge is also present on the hydrogen atom and it is acidic.
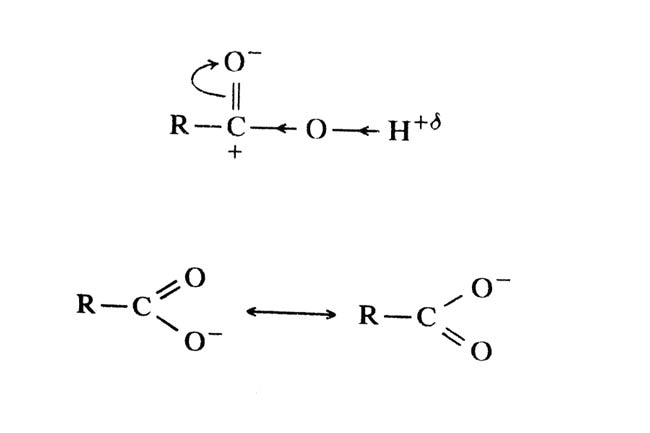
Carboxylic ion is obtained by dissociation of H+, which is stabilized by resonance. Each C–O bond has the same length in carboxylate ion due to resonance.
The acid power of carboxylic acids depends on the electron displacement properties of group R. If group R has the property of displacing electrons on its own, then this acid will be a strong acid.
If group R has the property of displacing electrons from the other side, this acid will be a weak acid. The +I effect of H, CH3, C2H5 and C3H7 groups increases in this sequence.
Therefore, the ability of these groups to displace electrons from themselves increases in this order. Therefore, the decreasing order of acid power of these acids is also the same.
HCOOH > CH3COOH > C2H5COOH > C3H7COOH
Same Here:
FCH2COOH > ClCH2COOH > BrCH2COOH > ICH2COOH
CCl3COOH > CHCl2COOH > CH2ClCOOH > CH3COOH
Derivatives of Carboxylic Acid
Acid Halides
The compound that is obtained when a -OH group of the -COOH group of a carboxylic acid is replaced by a halogen atom. It is called a carboxylic acid halogen or acyl halide. Carboxylic acid halide is abbreviated simply as Halide Acid.
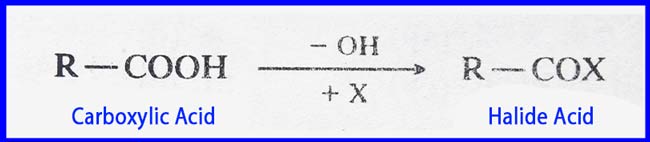
R–CO– group is called Acyl group. To make the name of Acid Halide in the normal method, remove ‘ek acid’ from the end of the name of the acid and put ‘el halide’.
To name them in the IUPAC method, they add ‘ol chloride’ to the end of their corresponding alkene name. Corresponding alkane’s english name removes ‘e’ from the end and adds ‘amide’. Following are the common and IUPAC names of some acid halides.
Acid Anhydrides
The compound obtained by the release of one molecule of water as a result of the interaction of both carboxylic group of two molecules of a mono carboxylic acid is called a carboxylic acid anhydride.
The compound obtained by the release of one molecule of water as a result of the interaction of two carboxylic groups present in the same molecule of a carboxylic acid is also called a carboxylic acid anhydride. Carboxylic acid anhydrides is also abbreviated as anhydride acid only.
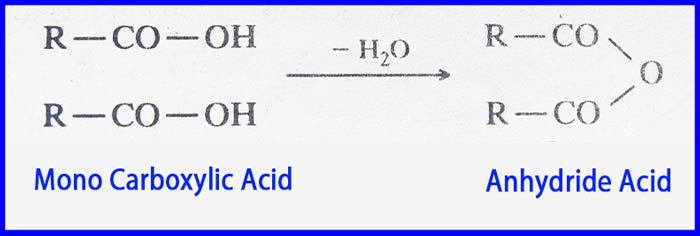
Anhydride acid is named after the name of the corresponding acid. Adding anhydride removes the acid from the end of the corresponding acid name. Following are the common and IUPAC names of some anhydride acids.
Amides acid
The compound obtained by substituting the -OH group of the -COOH group of a carboxylic acid with the -NH2 group is called a carboxylic acid amide. Carboxylic acid amide is also simply called amide acid.

In the ordinary method, they are named by removing ‘ek acid’ from the end of the corresponding acid and adding ‘amide’.
In the IUPAC method, they are named by adding amide at the end of the corresponding alkane name. Corresponding alkane’s English name removes e from the end and adds amide. Following are the common and IUPAC names of some amide acids.
Esters
Just as acid and base are made to make salt and water by acting, similarly acid and alcohol are made by making ester.
Example:
HCl(Acid) + NaOH(Base) → NaCl(Salt) + H2O(Water)
CH3COOH(Acid) + C2H5OH(Alcohol) → CH3COOC2H5(Ester) + H2O(Water)
The formation of ester by the action of an acid and an alcohol is called esterification. The acid used in this action can be any type of acid inorganic or organic.
Example:
C2H5OH(Alcohol) + H2SO4(Inorganic) → C2H5HSO4(Ester) + H2O
C2H5OH(Alcohol) + CH3COOH(Organic) → CH3COOC2H5 + H2O
An ester is obtained when a molecule of water is separated by the reaction of -COOH and -OH group in the reaction of a carboxylic acid and an alcohol.
Thus an ester is called carboxylic ester. This reaction is often carried out in the presence of concentrated sulfuric acid. In this reaction, concentrated sulfuric acid acts as both catalyst and dehydrator. The water molecule is formed by combining -OH of -COOH and -H of -OH.
The common formula of carboxylic ester is RCOOR’. To name it in the IUPAC method, remove the ‘oic acid’ from the end of the corresponding acid name and add ‘Oate’ and thus write the name of the alkyl group present in the first ester group. Following are common and IUPAC names of some carboxylic ester.
What is the Difference between Ester and Salt