Catalysts: Theories of catalysis and Uses of catalysts
Characteristics of Catalysts![]()
The important characteristics of catalysts or catalysis are the following:
Inactivity of catalyst
In the reaction of catalysis there is no change in the amount of catalyst and its chemical organization. There can be little change in physical form only.
Example: In the reaction of making oxygen from potassium chlorate(KClO₃), manganese dioxide is used as catalyst. The physical state of manganese dioxide is solid. As a result of the reaction, the size of its particles decreases, that is, it is converted from coarse particles into fine powder but there is no change in its quantity and chemical organization.
Subtle quantity of catalyst
Generally, only a small amount of catalyst is able to increase or decrease the speed of the catalysis reaction.
Example: Only 1 gram of colloidal platinum is sufficient to speed up the decomposition of large amounts of 106 liter of hydrogen peroxide.
There are some exceptions to this feature of catalyst. In some homogeneous catalysis reactions, the speed of the reaction is proportional to the amount of catalyst. Similarly in some heterogeneous catalysis reactions, the increase in reaction speed is proportional to the growth of the bottom of the catalyst.
Catalyst does not initiate any reaction
catalyst does not initiate any reaction. It can only increase or decrease its speed. Some scientists are of the opinion that catalyst can also initiate the reaction. In relation to these reactions it can also be said that the reaction takes place even in the absence of catalyst but its speed is so low that it does not show any change in years and it seems that the catalyst initiates the reaction. .
The vatalyst does not affect the nature of the product of the reaction
Generally, catalyst does not change the nature of the products of the reaction.
Example: The combination of nitrogen and hydrogen will always produce ammonia regardless of whether the catalyst is used or not.
There are some exceptions to this property of catalyst, such as – the reaction of hydrogen and carbon monoxide produces different products in the presence of different catalysts.
CO + 3H2 – Ni → CH4 + H2O
CO + 2H2 – ZnO+Cr2O3 → CH3OH
CO + H2 – Cu → HCHO
The action of vatalyst is specific
The action of Catalyst is specific. This means that a single substance can act as a catalyst for a particular reaction. It is not necessary that it also acts as a catalyst for other reactions. The best example of this is enzymes. An enzyme can participate in only one type of action. When making alcohol from starch, different enzymes are used as catalyst for each term.
Starch → Maltose → Glucose → Alcohol + CO2
Diatase, Maltase and Zymase enzymes catalyst in the above reactions.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
There are many substances that can catalyze many reactions. Example: Transition metals such as iron, kobalt, nickil, platinum etc. are used as catalysts in many reactions but each catalyst cannot be used for each reaction.
Effect of heat on catalyst
The efficiency of each catalyst is maximum at a certain temperature. This temperature is called optimum temperature.
The catalyst does not change the equilibrium
The catalyst does not change the equilibrium of a reversible reaction. It affects forward and backward reactions equally. The presence of unfathomable catalysts helps to establish equilibrium quickly but does not alter the equilibrium.
The effect of a catalyst on equilibrium can be explained based on the Arrhenius equation and the progress of the reaction.
According to the arrhenius equation:
K = A.e–E/RT
The value of activation energy (E) is reduced in the presence of catalyst. Hence, the value of the velocity constant (k) increases. Taking the logarithm of the above equation:
In k = In A – E/RT
log k = log A – E/2.303 RT
log k1 = log A1 – E1/2.303 RT
log k2 = log A2 – E2/2.303 RT
log k1 – log K2 = log A1 – log A2 – (E1 – E2 / 2.303 RT)
log k1/k2 = log A1/A2 + ΔE/2.303 RT
log K = log A1/A2 + ΔE/2.303 RT
Where K is Equilibrium constant.

In the presence of catalyst the values of E1 and E2 decrease but the value of ΔE remains unchanged. Hence the value of K also remains unchanged. Since there is no change in the initial concentrations of the reactants, there is no change in the concentrations of the reactants and products at equilibrium, that is, the equilibrium remains unchanged.
Theories of Catalysis
Several theories have been presented from time to time to clarify the mechanism of catalysis, but none of them is such that all types of catalysis reactions can be elucidated. The mechanism of catalysis in a reaction is explained by one of the following theories.
Intermediate compound theory
Most catalysis reactions are interpreted on the basis of this theory. According to this theory, the mechanism of reaction changes in the presence of catalyst. This means that the steps used in the reaction are changed and the catalyst joins one of them.
In short, it can be explained by the following common example –
Suppose two substances A and B combine to form AB and the reaction speed is very low.
A + B → AB
The velocity of this reaction is increased in the presence of catalyst (X). According to the secondary compound theory, in this reaction, the first catalyst reacts with one of the reactants to form a secondary compound. The speed of combination of reactant and catalyst is greater than the speed of reciprocal combination of reactants.
A(reactant) + X(catalyst) → AX(secondary compound)
Secondary compound is temporary. It reacts with other reactants to form products and the catalyst is liberated.
AX + B → AB + X
Example:
(1) Diethyl ether is obtained by heating 1 ethyl alcohol with concentrated sulphuric acid at about 140°C. H2SO4 acts as a catalyst in this reaction. The mechanism of this reaction can be written as follows.
C2H5OH + H2SO4 → C2H5HSO4 + H2O
C2H5HSO4 + C2H5OH → C2H5OC2H5 + H2SO4
(2) In the sis chamber method of manufacturing sulfuric acid, nitrogen oxides act as catalyst. According to barjilius the mechanism of this reaction is the following.
O2 + 2NO → 2NO2
NO2 + SO2 → SO3 + NO
SO3 + H2O → H2SO4
Following is the mechanism of this reaction according to lunge.
NO + NO2 → N2O3
2SO2 + H2O + N2O3 + O2 → 2HSO4.NO
2HSO4.NO + H2O → 2H2SO4 + N2O3
Adsorption Theory
This theory was given by Faraday in 1833. The mechanism of mainly solid catalysts is explained with the help of this theory.
Accordingly, reactive substances are adsorbed at the bottom of the catalyst, which increases the local concentration of the reactants. Since the reaction speed is proportional to the concentration of the reactants, the reaction speed increases in the presence of catalyst.
As the reacting material participates in the reaction, they leave the surface of the catalyst and other molecules of the reacting substance are absorbed on the surface of the catalyst and thus the reaction proceeds.
Modern adsorption theory
The first two theories are coordinated in this theory. Before understanding this theory, it is necessary to understand adsorption.
Adsorption is the action of the concentration of another substance on the surface of a substance. The substance at the bottom of which another substance is concentrated is called adsorbent and this other substance is called adsorbate. Since gases have no bottom, they cannot act as adsorbents. Mainly solids act as adsorbent. The adsorbate material is mainly gases.
There are two types of adsorption:
a: Physical Adsorption
b: Chemical Adsorption.
In physical adsorption, the molecules of adsorbate are attached to the bottom of the adsorbent by attraction of physical forces or wander wall forces.
This attraction is weak. In chemical adsorption, the atoms of the adsorbate are joined to the atoms at the bottom of the adsorbent by chemical bonds.
All the valences of the atoms inside the adsorbent are complete. The atoms at the bottom of the adsorbent do not have all their valences.
These are called free valences. Due to the free valence at the bottom of the adsorbent, a chemical bond is formed between the adsorbent and the adsorbate atoms.
The valences of the bottom and inner atoms of a solid (Z) are shown in the figure. The atoms inside the solid are connected to other atoms, their valences are fully satisfied, and their valences have been shown by solid lines. Some of the valences of the atoms at the bottom of the solid are not satisfied and these free valences are represented by dotted lines.
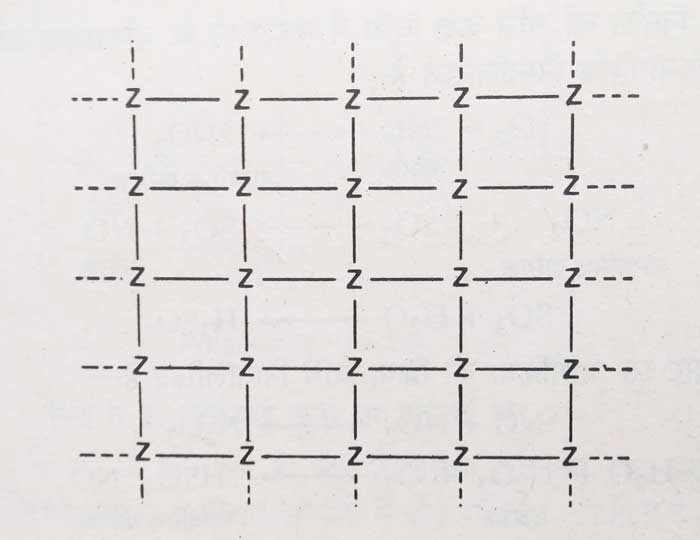
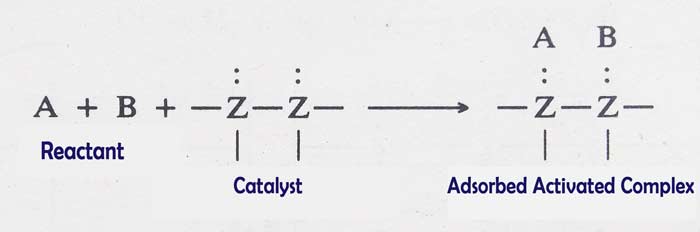
According to modern adsorption theory –
- The first reacting molecules adsorbate at the bottom of the catalyst by physical adsorption, which increases their local concentration.
- The reactant molecule makes a temporary coincidence with the free valences available at the bottom of the catalyst.
- The molecules that pass close to the bottom of the adsorbent form an adsorbed activated complex.
- The internal energy of the adsorbed activated complex is very high. It decomposes instantly and produces the product.
- The product separates from the bottom of the catalyst. After this, the other reacting molecules adsorbate at the bottom of the catalyst and proceed the reaction by repeating the same sequence.
From the above description, it is clear that free valences are the basis of activation of catalyst. Therefore, the more free valences in any catalyst, the more functional it will be. The increase in the number of free valences can be increased by the subdivision of the catalyst into the rough surface of the b catalyst.
The fragments obtained from the subdivision of the solid (Z) fragments are shown in figure. It is clear that the number of free valency increases from 20 to 34. Therefore, the sub-division of catalyst can increase its free valency and increase its efficiency.
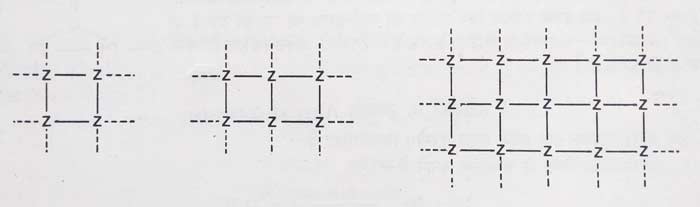
By x ray analysis it has been found that the bottom of any catalyst is not flat, but rather rough and has small cracks. At such places, there are a large number of free connectives. The efficiency of the catalyst is also greater when the number of free valences is higher. These rough and cracked places where the catalyst has high reactivity. This is called active centres.
- How p-n Junction Diode works : Forward and Reverse Biasing
- Semiconductors : How Semiconductor works and Types
- X-Rays – Production, Properties, Wavelength and Uses
- Daily use Chemical Compounds and Their Properties
- Hard Water and Soft Water : Permutit and Anion Exchange Resins
Based on modern adsorption theory, the functionality of colidy catalysts, the mechanism of catalysts inhibitors and the mechanism of catalysts poison can be clarified.

Uses of Catalysts
Some of the major catalyst and their major uses are as follows.
Iron: From haber method to making ammonia
N2 + 3H2 –Fe+Mo→ 2NH3 (500°C – 200atm)
In this reaction, molybdenum acts as a positive catalysts.
Nickel: in the manufacture of vegetable ghee from vegetable oil –
Vegetable oil + H2 –Ni→ Vegetable ghee
Platinum: in the manufacture of sulphuric acid by the contact method –
2SO2 + O2 → 2SO3
SO3 + H2O → H2SO4
In the manufacture of nitric acid by the ostwald method –
4NH3 + 5O2 → 4NO + 6H2O
2NO + O2 → 2NO2
3NO2 + H2O → 2HNO3 + NO
Iron oxide: to make hydrogen from water gas
CO + H2 + H2O → CO2 + 2H2
Nitrogen oxide: in the manufacture of sulphuric acid by sis chamber method –
2SO2 + O2 → 2SO3
SO3 + H2O → H2SO4
Cupric chloride: in the manufacture of chlorine from the Deacon method –
4HCl + O2 → 2H2O + Cl2
Alumina: in the manufacture of diethyl ether –
2C2H5OH → C2H5OC2H5 + H2O
Concentrated sulphuric acid:
1) in the manufacture of diethyl ether –
2C2H5OH → C2H5OC2H5
+ H2O
2) Esterification and water decomposition of esters –
CH3COOH + C2H5OH → CH3COOC2H5 + H2O
The mixture of oxides of cobalt, thorium and magnesium is used as catalyst in the manufacture of synthetic petrol.
The enzyme catalyst participates in many biochemical reactions.
