What is Deuterium or Heavy Water? How to make Deuterium?
Oxygen of deuterium (heavy hydrogen) is called heavy water (D2O) . It is also called deuterium oxygen. Ordinary water contains about 1 part of heavy water in 6000 parts.
Heavy water was discovered by Urey in 1932. It was first made by Macdonald in 1933 by electrolysis of ordinary water by concentrating. Urey was awarded the Nobel Prize in 1934 for the discovery of deuterium and heavy water.
Deuterium (Heavy Water)
Heavy Water Preparation
Burning of deuterium – By burning deuterium in air, heavy water is obtained.
2D2 + O2 → 2D2O
It is also obtained by exchange reaction of deuterium with simple water.
H2O + D2 → 2D2O + H2
By fractional distillation of ordinary water – Bbout 1 part of heavy water in 6000 parts of ordinary water.
Heavy water can be separated from ordinary water by fractional distillation. For this, about 1200 cm long fractional column is used. The boiling point of heavy water (D2O) is higher than that of light water (H2O).
Therefore, by charge distillation of ordinary water, light water is obtained in the distillate and heavy water in the residue.
Electrolytic Method
Electrolytic method – Heavy water can also be separated from ordinary water by electrolysis of ordinary water. (Uery 1932) found that the electrolysis of light water (H2O) is about six times faster than the electrolysis of heavy water.
Therefore, on electrolysis of ordinary water, the proportion of heavy water in it gradually increases.
Are there any methods to obtain heavy water by electrolysis of ordinary water. Among these, the method developed by Taylor, Eyring or Frost is used today for industrial production of heavy water.
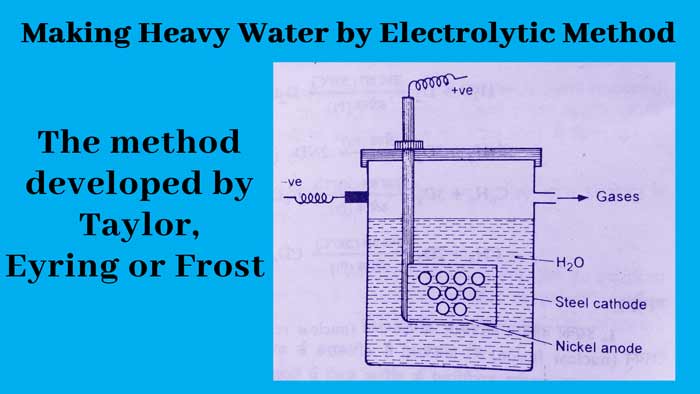
In the electrolytic cell used in this method, an iron vessel acts as the cathode and the anode is made of nickel. First, the electrolysis of N/2 solution of NaOH in ordinary water is done.
About one-fourth of the absolute volume remains, then after distilling it, adding NaOH to the distillate, its concentration is again reduced to N/2 and electrolysis is done again. After repeating this process six times, the water obtained is almost 100% heavy water.
Physical Properties
Like ordinary water, heavy water is also colourless, odorless and tasteless. There is a difference between it and the physical constant of light water (H2O). The boiling point and melting point of light water are 100°C and 0°C respectively while the boiling point and melting point of heavy water are 101.42°C and 3.8°C respectively.
Its density at 20°C is 1.1059 g per cm3 which is 11% higher than the density of light water (0.998 g per cm3) at this temperature. Light water has a maximum density of 4 c while heavy water has a maximum density of 11.6°C. Its molecular weight is 20.
Chemical Properties
It is similar to light water in chemical properties, but the speed of chemical reactions of heavy water is often half of the speed of chemical reactions of light water. Some of its main chemical properties are as follows.
1. Electrolysis
In the presence of a small amount of an electrolyte, the following reaction takes place when it is electrolysis.
2D2O → 2D2 + O2
2. Reaction with Sodium
It reacts with sodium and other reactive metals to form deuterium gas.
2Na + 2D2O → 2NaOD + D2
3. Action with Oxacido
By reacting with acidic oxido, it reacts with acid and phosphoric oxides to form base –
Na2O + D2O → 2NaOD (Sodium Deuteroxide)
N2O5 + D2O → 2DNO3 (Deutero-Nitric acid)
SO3 + D2O → D2SO4 (Deutero-sulphuric acid)
4. It reacts with nitrides to form heavy ammonia (ND3).
Mg3N2 + 6D2O → 3Mg(OD)2 + 2ND3
5. It reacts with carbides to form deuterium containing hydrocarbons.
Al4C3 + 12D2O → 4Al(OD)3 + 3CD4 (Deutero Methane)
CaC2 + 2D2O → Ca(OD)2 + C2D2 (Deutero Acetylene)
Exchange Reactions
When hydrogen containing compounds are reacted with heavy water, hydrogen and deuterium are exchanged.
The replacable hydrogen present in the hydrogen-containing compound, that is, the hydrogen on which there is a partial or full positive charge, is easily displaced by deuterium.
The exchange of ionized hydrogen and deuterium takes place very slowly and a catalyst is required for this process.
HCl + D2O → DCl + HOD
NaOH + D2O → NaOD + HOD
NH4Cl + 4D2O → ND4Cl + 4HOD
CH3NH2 + 2D2O → CH3ND2 + 2HOD
CH3COOH + D2O → CH3COOD + HOD
Biological Properties
Small amount of heavy water present in natural water is not harmful for living organisms (plants and animals) but pure or concentrated heavy water is harmful for living organisms. The use of pure heavy water stops the growth of many living beings.
The reactions of heavy water take place at a slower rate than the reactions of ordinary water. Due to this, the use of heavy water leads to imbalance in the biochemical reactions and it is harmful to the living beings.
- What is Deuterium or Heavy Water? How to make Deuterium?
- Nuclear Reactions with Examples : Nuclear Fusion and Fission with Example
- Theories of reaction rates: Chemical Kinetics
- Bohr-Bury Scheme | Bohr and Bury Rule | Chemistry Notes
- Bohr's Atomic Model: What is Bohr's model of an atom? chemistry Notes
It is used as a moderator in nuclear reactors.
It is used to make heavy hydrogen (deuterium) and heavy hydrogen compounds (ND3, CD4 C2D2 CH3COOD, D2SO4).
It is also used to study the mechanisms of various reactions and processes.
