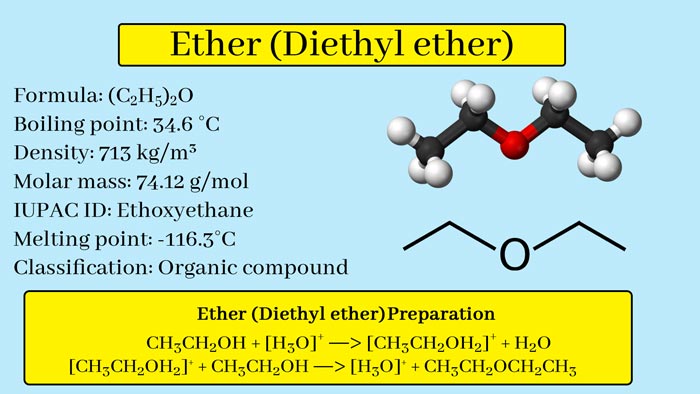
Diethyl Ether: How Diethyl Ether is prepared | Uses | Properties
It is the dominant member of the ether class. It is also called simply ether or ethyl ether.
Methods of Preparation
By the action of Iodoethane and dry silver oxide: Diethyl ether is obtained by heating ethyl iodide with dry silver oxide.

Williamson Synthesis Method: In this method sodium or potassium ethoxide is heated with chloro ethane, bromo ethane or iodo ethane. This reaction results in Diethyl ether.
Read more about Williamson Synthesis Method…
C2H5ONa + C2H5I → C2H5 – O – C2H5 + NaI
By heating a mixture of ethyl alcohol and alumina: heating ethyl alcohol with alumina (Al2O3) at 250°C gives diethyl ether.
C2H5OH + HOC2H5 – Al2O3/250°C→ C2H5 – O – C2H5 + H2O
Laboratory Method:
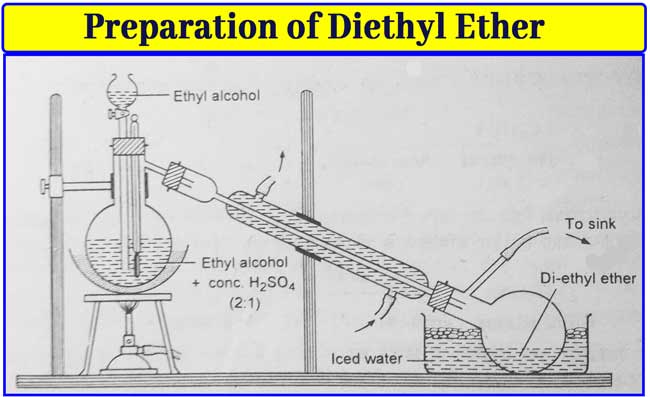
By heating a mixture of ethyl alcohol and sulphuric acid: Diethyl ether is obtained by heating this mixture to about 140°C by mixing a high concentration of ethyl alcohol with slightly concentrated sulfuric acid.
C2H5OH + H2SO4 → C2H5HSO4 + H2O
C2H5HSO4 + C2H5OH → C2H5 – O – C2H5 + H2SO4
It is clear from the above reaction that a small amount of concentrated sulfuric acid taken initially can convert a very high amount of alcohol into ether. Hence this method is also called Continuous etherification method.
In practice this does not happen. Because some sulfuric acid gets reduced to form sulfur dioxide and the water concentrate in the reaction dilutes sulfuric acid. Therefore small amounts of acid are added from time to time.
About 100 ml of pure alcohol is taken in a 500 ml distillation flask. About 50 ml of concentrated sulfuric acid is slowly added to it. While adding sulfuric acid, shake the flask. Now heat the flask to 140°C by placing it on sand heat.
The ether is distilled and collected in the receptor. The receiver has a side-tube which is connected to a rubber drain and reaches the water sink. This does not allow the ether vapor to reach the burner and there is no fear of fire.
All cork in the equipment is air tight. The speed with which the ether collects in the receiver keeps pouring the alcohol into the flask from the point funnel accordingly.
- How Transistor Works : PNP and NPN Transistors
- How p-n Junction Diode works : Forward and Reverse Biasing
- Semiconductors : How Semiconductor works and Types
- X-Rays – Production, Properties, Wavelength and Uses
- Daily use Chemical Compounds and Their Properties
- Hard Water and Soft Water : Permutit and Anion Exchange Resins
- Properties of Water :- Purification, Sources and Uses
- Hydrogen Chloride : Preparation, Properties and Uses
Rectification: Alcohol, water and sulfuric acid are present in the form of impurities in ether obtained from the above method. To remove sulfuric acid, the obtained ether is washed with a solution of sodium dioxide.
To remove alcohol, ether is mixed with 50% calcium chloride solution and left for some time, forming crystals of CaCl2.3C2H5OH. They are filtered and separated.
To remove water, it is dried by adding anhydrous calcium chloride to the ether, thus distilling the obtained ether at 35°C to obtain pure ether.
Diethyl Ether Physical Properties
It is a colorless, pungent and highly volatile liquid. It burns with a light flame. Its boiling point is 34°C. It is less soluble in water but less soluble in organic solvents such as alcohol benzene chloroform etc.
It is itself used as a solvent. By correcting its vapors, there is unconsciousness. More heat is used than its evaporation. Therefore, applying on any part of the body makes that part cool. That is, it becomes numb for some time.
Diethyl Ether Chemical Properties
Ether is a relatively inert substance. They do not react with alkalis, dilute acids, reducing oxidizers and active metals at ordinary temperatures.
Following are the major chemical reactions of diethyl ether.
Combustion: Due to being more flammable it burns quickly and forms an explosive mixture with air.
C2H5 – O – C2H5 + 6O2 → 4CO2 + 5H2O
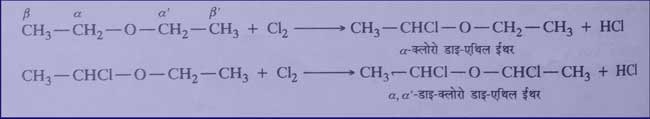
Halogenation: It reacts with chlorine and bromine to form substitution products. Substitution on α-carbon atoms is facilitated i.e. hydrogen is substituted first from oxygen to carbon atom and then other hydrogen atoms are substituted depending on the amount of the agent and other conditions.
Produce oxonium salt: It reacts with cold and concentrated H2SO4 or HCl to form oxonium salt.
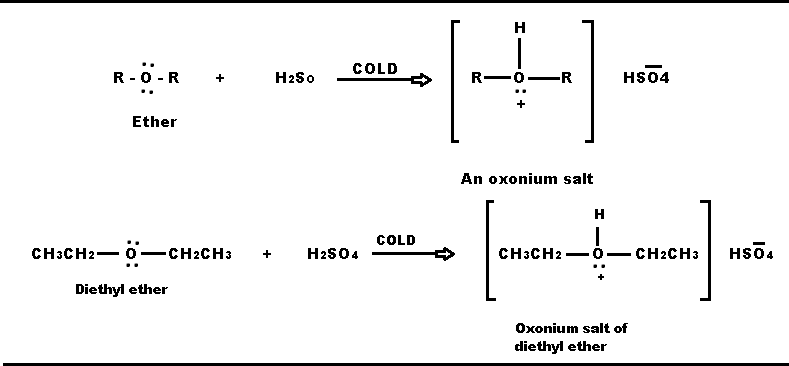
In this reaction, the H+ of the acid is combined with a lone pair of electron on oxygen. This reaction takes place in the same way as ammonium salts are formed by the reaction of ammonia or amino compounds and acids.
Therefore, ether is soluble in cold and concentrated H2SO4. With the help of this property of ethers, ethers can be differentiated into alkens. The elken is insoluble in cold and concentrated H2SO4.
Ether can be reclaimed by adding water to oxonium salt.
Reaction with concentrated sulfuric acid: Ethyl hydrogen sulfate is obtained by heating the diethyl ether with concentrated sulfuric acid.
C2H5 – O – C2H5 + H2SO4 → C2H5HSO4 + C2H5OH
C2H5OH + H2SO4 → C2H5HSO4
Over heating, 160 ethylene gas is obtained.
Water decomposition: Water decomposition of the ether occurs when heated with dilute sulfuric acid at high pressure. Water decomposition of diethyl ether yields ethyl alcohol.
C2H5 – O – C2H5 + H2O → 2C2H5OH
Formation of peroxide or auto oxidation: In the presence of sunlight, reaction of diethyl ether with air, oxygen or ozone is made of peroxide.
ether’s peroxide is an extremely explosive substance. Therefore, more careful action is required in the distillation of the ether, by removing the detergent like FeSO4 with a reducing agent to remove the peroxide present in it.
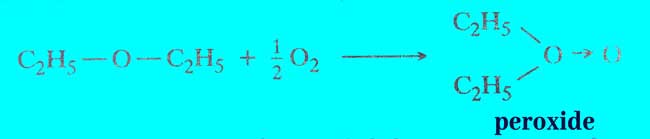
Reaction with Phosphorus Penta Chloride: Ethyl chloride is formed when the diethyl ether reacts with phosphorus penta chloride.
C2H5 – O – C2H5 + PCl5 → 2C2H5Cl + POCl3
Reaction with hydroiodic acid: diethyl ether with cold and concentrated hydroiodic acid forms ethyl iodide and ethanol.
C2H5 – O – C2H5 + HI → C2H5I + C2H5OH
It forms ethyl iodide with hot and concentrated HI.
diethyl ether is a simple ether (R – O – R). The reaction of mixed ether(R1 – O – R2) with cold and concentrated HI can occur in two ways.
R1 – O – R2 + HI → R1OH + R1I
R1 – O – R2 + HI → R1I + R2OH
It is known from the detailed study of the mechanism of the above reaction. That if one of the alkyl groups forms a more stable carbonium ion then its iodide is formed.
(CH3)3C – O – CH3 + HI → (CH3)3Cl + CH3OH
In other circumstances the small alkyl group forms iodide compounds.
CH3 – O – C2H5 + HI → CH3I + C2H5OH
Reduction: Diethyl ether is reacted with red phosphorus and hydroiodic acid and gets ethane.
C2H5 – O – C2H5 + 4H → 2C2H6 + H2O
Oxidation: Reaction with strong oxidants such as acidic potassium dichromate makes it acetaldehyde and acetic acid.
C2H5 – O – C2H5 + 2O → 2CH3CHO + H2O
CH3CHO + O → CH3COOH
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
Reaction with acetyl chloride: On heating with acetyl chloride in the presence of anhydrous zinc chloride, it forms ethyl chloride and ethyl acetate.
C2H5 – O – C2H5 + CH3COCl → C2H5Cl + CH3COOC2H5
Reaction with acetic anhydride: The reaction with acetic anhydride of diethyl ether is similar to that of acetyl chloride.
C2H5 – O – C2H5 + (CH3CO)2O → 2CH3COOC2H5
Reaction with carbon mono oxide: In the presence of boron tri chloride at about 500 atmospheric pressure and about 100°C temperature it reacts with carbon mono oxide to form ethyl propanoate.
C2H5 – O – C2H5 + CO → C2H5 – CO – O – C2H5
Effect of heated alumina: Ethylene gas is obtained when the vapors of diethyl ether flow at 360°C over the heated alumina.
C2H5 – O – C2H5 → 2C2H4 + H2O
Diethyl Ether Uses
It is used as a solvent and medium in making Grignard reagents and in the Burtz reaction.
Ether has been used in surgery as an anesthetic in combination with chloroform.
By mixing ether in alcohol and used as a fuel in place of petrol like Power alcohol.
It is also used as a refrigerant.
The major use of ether is as a solvent. It easily dissolves many organic substances like oils, fats and resins.