Discovery of Nucleus of the Atom and Moseley experiment
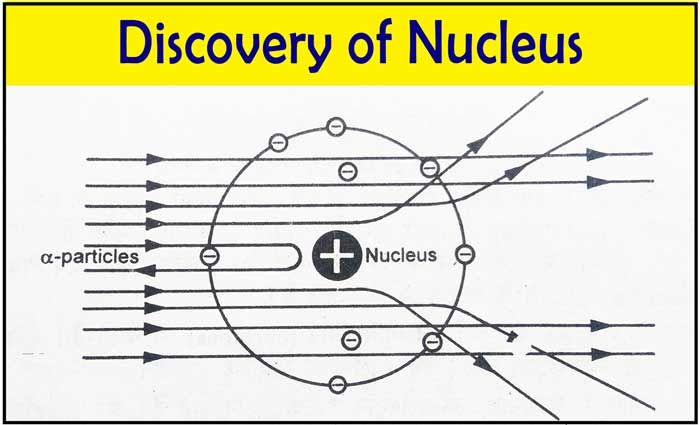
E Rutherford discovered the nucleus of an atom in 1911. Geiger and Marsden, the scientists working under the direction of Rutherford, used bombardment of alpha particles on a thin sheet (4 x 10-5 cm) of metals such as gold, silver, etc.
 Discovery of Nucleus
Discovery of Nucleus
In these experiments, α-Particles obtained from uranium move only in one direction as a beam. The lead block absorbs radiation from other directions. The α-Particles collide with metal foil, moving in straight lines.
A fluorescent screen is placed around the metal foil. Scattering of α-Particles is done by metal foil. Due to this scattering, after coming out of the metal foil, the α-Particles collide at different places on the screen and produce glow.
Thus the number of particles that deviated while coming out of the metal foil was estimated.

In the above experiments, it was found that there is no deviation in the path of most α-Particles from the metal foil. Some α-Particles deviate from their passage as they pass through the metal sheet. Out of about 20000 α-Particles, only one α-Particles deviates from an angle of 90° or more.
How to write Electronic Configuration of Atom | Arrangement of orbit & orbitals in atoms
https://www.youtube.com/watch?v=4FBQxYeMTfU
Rutherford made the following conclusions from experiments of scattering of α-Particles by a thin sheet of metal.
- There is no deviation in the path of most α-Particles from the metal foil. Hence, most of the space in an atom is empty.
- Some α-Particles repel with metal foil and return. Hence, it proves that there are some positively charged and heavy particles in the atom.
- The number of particles whose path deviates is very small. Therefore, the space encircled by the positively charged and heavy particles is very less.
- On the basis of the above experiments and conclusions, Rutherford suggested that almost the entire weight and total positive charge of the atom is located in a microscopic volume at its center. This part of an atom is called its nucleus.
Atoms of different elements have different sizes. The radii of atoms are between 0.37A° and 2.62 A°. In other words, the radii of atoms are in the range of 10-8 cm. The radius of the nucleus is in the range of 10-12 cm.
It is clear that the radius of an atom is approximately 10,000 times greater than the radius of the nucleus, the volume of the atom is 1012 times greater than that of the nucleus. Therefore, the volume of an atom is much larger than the volume of its nucleus.
An example of the comparison of atomic and nucleus sizes is this – the radius of the football field is about 50 meters and the radius of the head of an allpin is 0.5mm.
The same ratio as the radius of the atom and nucleus. Therefore, if an allpin is buried in the center of a football field, then the head of the allpin can be considered as the nucleus equivalent and the football field is equivalent to the atom.
The radii of all the atoms, nuclei and atomic particles of the atom can be determined by experiments based on x-rays. The radius of the electron is 2.8 x 10-18 cm.
The radius of the proton is 10-13. The radius of the neutron is also 10-13 cm. The radius of the nucleus of an atom is proportional to the plus root of the number of nucleons present in it.
r = 1. 33 x 10-13 x A1/3
r = radius of nucleus
A = number of nucleons
The proton and neutron are heavy particles and the proton is positively charged. Therefore, in an atom, all protons and neutrons are located in its nucleus and electrons reside outside the nucleus. The atomic particles of an atom are also obtained from its nucleus.
The atomic particles of an atom are not permanently located in its nucleus but are obtained as a result of the transformation of matter and energy in the process of nuclear dissolution.
The particles present in the nucleus of an atom are also called nucleons. Since the nucleus of an atom consists of proton and neutron, nucleons are the collective name of proton and neutron.
An atom of hydrogen usually contains an electron and a proton. Therefore, there is only one proton in the nucleus of hydrogen. Therefore, there is no difference in a proton of hydrogen nucleus (H+) and the cation(H+) of hydrogen is also called proton.
The use of scattering of α-Particles of Rutherford does not yield any information about the number of protons and electrons present in the atom. The use of Mosley provides information about this.
- Discovery of Nucleus of the Atom and Moseley experiment
- Proton | Discovery, Mass, Charge | Chemistry Notes
- Electron: Discovery, Charge and mass of Electron
- Unsaturated Hydrocarbons: Different between Alkenes and Alkynes
- General Organic Chemistry Notes: Download chemistry Notes PDF file
- Chemical Bonding Notes: Class 11 Chemistry Revision Notes
- Classification of Elements and Periodicity in Properties Notes
- Surface Chemistry Notes: CBSE Notes Class 12 Download Short Note PDF
The particles present in the nucleus of an atom are also called nucleons. Since the nucleus of an atom consists of proton and neutron, nucleons are the collective name of proton and neutron.
An atom of hydrogen usually contains an electron and a proton. Therefore, there is only one proton in the nucleus of hydrogen.
Therefore, there is no difference in a proton of hydrogen nucleus (H+) and the cation(H+) of hydrogen is also called proton.
The use of scattering of α-Particles of Rutherford does not yield any information about the number of protons and electrons present in the atom. The use of Mosley provides information about this.
Moseley Experiment
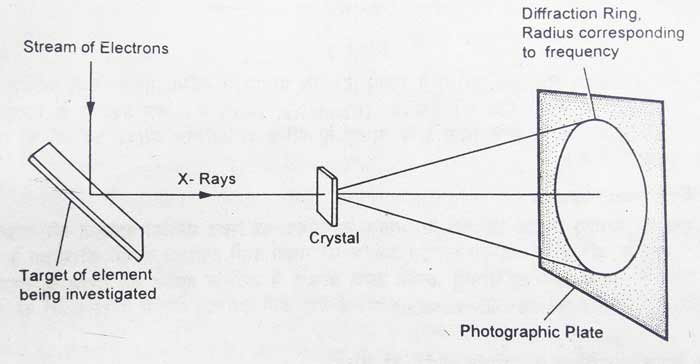
When cathode rays from the immersion tube collide with an element at a rapid speed, x-rays are obtained. x-rays were discovered by professor Rontgen. x-rays differ in many ways from cathode rays. They have high penetrating power and do not deviate from the electrical or magnetic field. Their wave length is in the order of 10-8 cm.
By placing a solid target in the path of cathode rays, x-rays are obtained from the solid target. The solid target is called anticathode and is a simple method of obtaining x rays. Diffraction of x-rays occurs when placing a crystal of potassium ferricyanide in the path of x-rays obtained from the solid target.
A photographic plate is placed in the path of x-rays when potassium ferricyanide is released from the crystal. As a result of diffraction, the x-ray spectrum of elements on the photographic plate are obtained as circular shapes. The frequencies of x-rays can be found from the radii of circular shapes.
Mosley observed that the frequency (v) of x-rays derived from different elements is thus related to the atomic number (Z), a characteristic property of those elements, as follows –
√v = a(Z – b)
Where a and b are two constant.
According to the equation √v = a known by Mosley, drawing a graph in √v and Z gives a straight line. Since drawing a graph between √v and atomic weight does not yield a straight line, the value of Z cannot be equal to the value of atomic mass.

Mosley did a detailed study of the x-ray spectrums of the elements and found the value of atomic number Z for different elements. It is found that the value of Z is an absolute integer in each case and is less than the value of atomic mass. Apparently, when drawing a graph between the values of atomic number Z determined by √v and mosley, a straight line is obtained.
Vander broeck (1913) suggested that the number of positive units present on the nucleus of an atom is equal to the atomic number. Chadwick proved with the help of Rutherford’s use of α-Particles scattering and other experiments that the number of positive units present on the nucleus of an atom is equal to atomic number z.