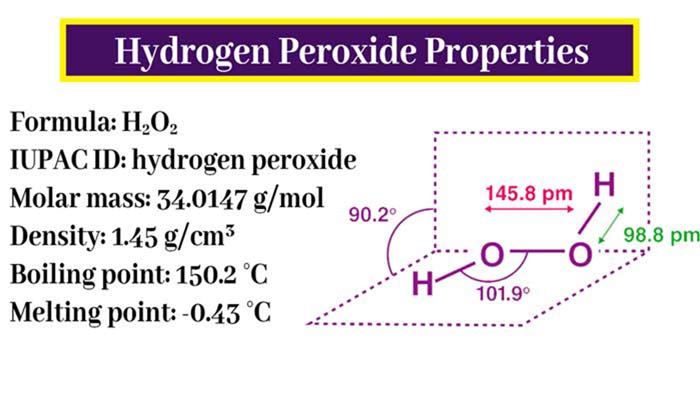
Chemical Properties of Hydrogen Peroxide
By the this methods (click here) a dilute solution of hydrogen peroxide is obtained, which is concentrated by the following method –The boiling point of hydrogen peroxide is 151°C. It decomposes on heating, decomposes rapidly at boiling point. Therefore, it is distilled under low pressure. Its boiling point is 68°C at 26 mm pressure. At a pressure lower than this, its boiling point is even lower.
- Preparation of Aldehydes and Ketones : Chemistry Page
- Arsenious Oxide : Preparation, Properties and Uses
- How to find Equivalent Weight in Chemistry ? Chemical Formula
- Oxidation Number : How to find Oxidation State
- Bleaching Powder : Preparation, uses of Bleaching Powder
Its dilute solution is distilled at about 26 mm of pressure to concentrate hydrogen peroxide. This pressure can be easily achieved with the help of a water pump. Hydrogen peroxide of about 50% concentration can be obtained by this method.
Hydrogen peroxide is distilled at about 14 mm of pressure for higher concentrations. Hydrogen peroxide of about 90% concentration is obtained by this method.

Hydrogen peroxide of 99% concentration is obtained by drying it in a vacuum desiccator at 90% concentration of concentrated H2SO4. To remove the final amount of water, H2O2 is cooled by placing it in a freezing mixture of solid CO2 and ether, from which it separates into a solid, which on heating gives Hydrogen peroxide of 100% concentration.

Chemical Properties
Decomposition: It is a temporary substance. In the presence of sunlight, heat, rough surface etc., it decomposes into water and oxygen.
2H2O2 → 2H2O + O2
Oxidizing agent: It accepts and discards electron. Hence it acts as both an oxidising agent and a reducing agent. The oxidation number of O in H2O2 is -1. The permanent oxidation numbers of O are 0 and -2.
Therefore, the oxidation number of O in H2O2 may increase or decrease in chemical reactions. Therefore, it can act as both an oxidising agent and a reducing agent. When the oxidation number of O in H2O2 becomes -1 and -2, it acts as an oxidising agent.
In its oxidising reactions, it provides nascent oxygen according to the following half reaction –
H2O2 → H2O + O2
Its major oxidising reactions are as follows –
1) Iodine is released from potassium iodide
H2O2 → H2O + O2
2KI + H2O + O2 → 2KOH + I2
so
2KI + H2O2 → 2KOH + I2
2) Oxidizes black colored lead sulfide to white colored lead sulfate.
[H2O2 → H2O + O2] x 4
PbS + 4O → PbSO4
PbS + 4H2O2 → PbSO4 + 4H2O
3) oxidizes ferrous sulfate [FeSO4] to ferric sulfate [Fe2(SO4)3] in the presence of dilute H2SO4.
2FeSO4 + H2SO4 + H2O2 → Fe2(SO4)3 + 2H2O
4) Oxidizes sulfurus and arsenius acid to sulfuric and orcenic acid.
H2SO3 + H2O2 → H2SO4 + H2O
H3AsO3 + H2O2 → H3AsO4 + H2O
5) It oxidises nitrites to nitrate, sulfites to sulfate and orsanits to orsanate.
example :
KNO2 + H2O2 → KNO3 + H2O
K2SO3 + H2O2 → K2SO4 + H2O
Na3AsO3 + H2O2 → Na3AsO4 + H2O
6) oxidizes acidic potassium ferocinide to potassium fericinide.
2K4Fe(CN)6 + H2O2 + H2SO4 → 2K3Fe(CN)6 + K2SO4 + 2H2O
7) In the presence of ferrous sulphate, it converts benzene to phenol.
C6H6 + H2O2 → C6H5OH + H2O
8) Converts formaldyhide to pharmic acid in basic solution.
2HCHO + H2O2 → 2HCOOH + H2
9) H2S gives out S.
H2O2 + H2S → 2H2O + S
Reducing agent: It reacts with any oxidising substance to form a sub-oxygen. In this process the oxidising number of O in H2O2 is reduced from -1 to 0. Hence it is also a waste. Its main reducing reactions are as follows –
It reacts with ozone to form oxygen.
O3 + H2O2 → 2O2 + H2O
In this reaction H2O2 is oxidised to O2 and H2O2 is reduced to H2O. Thus, in this reaction, H2O2 itself acts as both an oxidising agent and a reducing agent.
It reduces chlorine to HCl.
H2O2 + Cl2 → 2HCl + O2
It reduces KMnO4 to magnus salt in acidic medium.
5H2O2 + 2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 8H2O + 5O2
In neutral medium it converts KMnO4 to MnO2 and the solution becomes alkaline.
H2O2 + 2KMnO4 → KOH + 2MnO2 + 2O2
It reduces the oxides of silver and mercury to silver and mercury.
Ag2O + H2O2 → 2Ag + H2O + O2
HgO + H2O2 → Hg + H2O + O2
It reduces lead dioxide to lead mono oxide.
PbO2 + H2O2 → PbO + H2O + O2
Reduces MnO2 to magnus salt in acidic solution.
MnO2 + H2SO4 + H2O2 → MnSO4 + O2 + 2H2O
Reduces basic potassium fericinide to potassium ferocinide.
2K3Fe(CN)6 + 2KOH + H2O2 → 2K4Fe(CN)6 + 2H2O + O2
Sodium removes oxygen from hipochlorite.
H2O2 + NaOCl → NaCl + H2O + O2
Reaction with potassium dicromate: The reaction of potassium dicromate and hydrogen peroxide in etheric solution in the presence of concentrated sulfuric acid results in the formation of blue percromate (CrO5).
K2Cr2O7 + H2SO4 + 4H2O2 → 2CrO5 + 5H2O + K2SO4
Blue percromate (CrO5) has two peroxide bonds (- O – O -) and its structure formula is as follows –
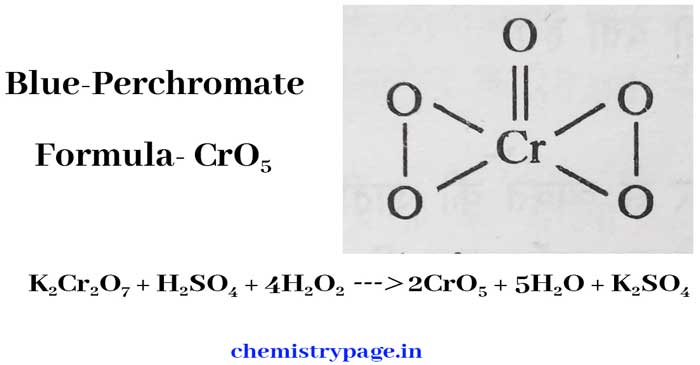
In both K2Cr2O7 and CrO5, the oxidation number of Cr is +6. Hence the above reaction is not a radox reaction. In the above reaction, K2Cr2O7 acts as an oxidising agent and H2O2 does not act as an oxidising agent or a reducing agent.
CrO5 is soluble in ether and is stable in ether solution. It is stable in aqueous solution and decomposes according to the following equation:
4CrO5 → 2Cr2O3 + 7O2
Acidic properties: Hydrogen peroxide reacts with bases to form salts. Hence it has acidic property.
2NaOH + H2O2 → Na2O2 + 2H2O
Na2CO3 + H2O2 → Na2O2 + H2O +CO2
Ca(OH)2 + H2O2 → CaO2 + 2H2O
Ba(OH)2 + H2O2 → BaO2 + 2H2O
Both its hydrogen are displaced. Hence it is a bi-phasic acid.
Bleaching property: It is a good oxidizer. Therefore, it spoils the color of many materials, such as silk, hair, etc. Its bleaching property is due to this reaction –
H2O2 → H2O + O