Introduction of Cannizzaro’s reaction || What is the Cannizzaro reaction used for?
Cannizzaro reaction ( Cannizzaro reaction) is an organic disproportionation reaction of an aldehyde without active hydrogen that undergoes intermolecular redox reaction under the action of a strong base. The Italian chemist Stanislaus Cannizzaro obtained benzaldehyde and benzyl alcohol by treating benzaldehyde with grass ash in 1895.
This reaction was first discovered, hence the name Cannizzaro reaction. Fatty aldehydes, aromatic aldehydes, or heterocyclic aldehydes that do not contain α-hydrogen atoms undergo simultaneous oxidation and reduction reactions of the aldehyde molecules under the action of concentrated bases to form the corresponding carboxylic acids (carboxylate salts in alkaline solutions) and alcohol Organic disproportionation.
Introduction
Italian chemist Stanisola Cannizzaro treated benzaldehyde with grass ash to obtain benzoic acid and benzyl alcohol. This reaction was first discovered, and the reaction name was derived from it. The reaction is essentially an organic disproportionation reaction between an aldehyde without α-hydrogen under the action of a strong base, a molecule of carboxylic acid and a molecule of alcohol.
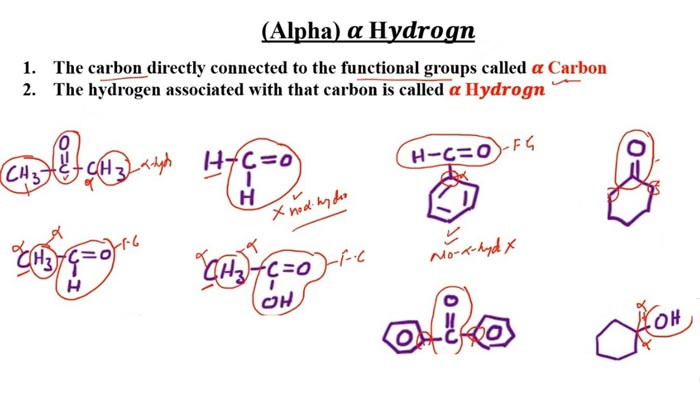
The aldehydes commonly used in the Cannizzaro reaction are aromatic aldehydes (such as benzaldehyde) and formaldehyde. For aldehydes with active hydrogen, alkali will capture active hydrogen, which will cause aldol reaction and reduce the yield of Cannizzaro reaction. The Cannizzaro reaction can also occur in the molecule, and sometimes the product hydroxy acid can be further dehydrated and cyclized to form lactones.
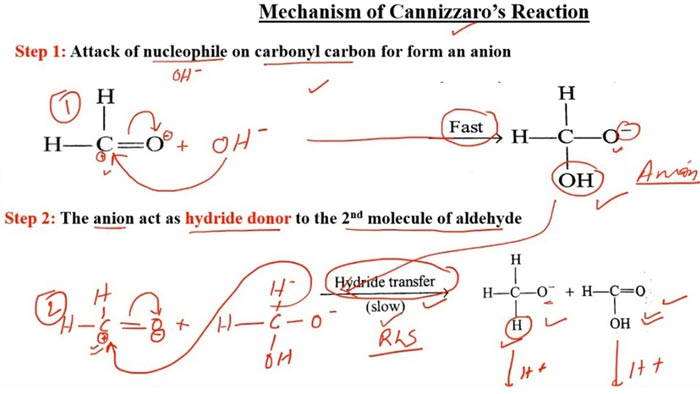
Reaction mechanism
Vanillin, p-hydroxybenzaldehyde, syringaldehyde, and formaldehyde are all aldehydes without active hydrogen. They undergo intra- and intermolecular oxidation-reduction reactions under the action of strong bases to form a molecule of carboxylic acid and a molecule of alcohol.
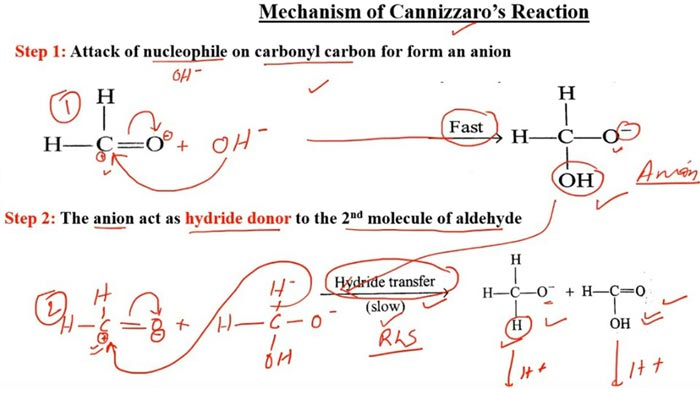
The nucleophilic addition of the base to the carbonyl occurs first, and the tetrahedral intermediate interacts with the strong base, losing a proton to a double negative ion (Cannizzaro intermediate). Because the oxygen atom has a negative charge and has a power supply, the ability of the adjacent carbon atom to repel electrons is greatly enhanced. Both anion intermediates can interact with aldehydes.
The hydrogen on the carbon carries a pair of electrons in the form of a hydrogen anion to the carbonyl carbon of the aldehyde, forming an alkoxide anion and a carboxylate anion. The water in the Cannizzaro reaction can participate in the reaction and generate hydrogen, which also confirms the negative hydrogen transfer process.
This reaction is a kinetic third-order reaction, a second-order reaction for aldehydes, and the first-order reaction for bases:
K = R & lt [RCHO]2 [OH –]
In a high-concentration alkaline environment, the second reaction process becomes dominant, and the secondary reaction to the base is now:
K = R & lt [RCHO]2 [OH – ] + K ‘[RCHO]2 [an OH]2
Cross Cannizzaro reaction
It is a type of Cannizzaro reaction. Mixing two different alpha-hydrogen-free aldehydes, such as formaldehyde and benzaldehyde, causes them to undergo a cross-reduction reaction under alkaline conditions. Since formaldehyde is the most reducible in aldehydes, it is always oxidized to formic acid by itself, and the other reactant is reduced to an alcohol. This method is used in the industrial preparation of pentaerythritol.
Chemical Reaction
Fatty Aldehyde
1. Aldehydes without alpha hydrogen atoms: formaldehyde produces methanol and formic acid, and glyoxylic acid produces glycolic acid and oxalic acid.
2. An aldehyde with an alpha hydrogen atom: This substance can form butyral aldehyde under appropriate conditions. Butanal can cross Cannizzaro reaction with the original aldehyde, as shown below:
3. Aldehydes with alpha hydrogen atoms in the presence of formaldehyde:
The resulting β-aldol can continue to cross Cannizzaro reaction:
Aromatic aldehyde
1. Mono-substituted benzaldehyde:
2. Disubstituted benzaldehyde:
① When there is at least one unsubstituted group at the ortho position of the formyl double bond, a normal disproportionation reaction proceeds.
② When formyl is halogen or nitro in the ortho position.
③ Tri- and tetra-substituted benzaldehyde.
④ Hydrolytic enzymes and hydrogenated North American berberine are generated.
Experimental conditions
1. Alkali concentration, aromatic aldehyde. 50% sodium hydroxide or potassium hydroxide, nitrobenzaldehyde 15 ~ 35%.
2. Solvent: alkaline water solvent.
3. Temperature: The temperature of the aldehyde-alkali mixture rises and
falls mildly, without strict temperature control. When the reaction is
too slow, it can be heated in a water bath until the reaction is
completed. For nitrobenzaldehyde reacted at a 35% base concentration,
the temperature needs to be controlled at 45 ° C, otherwise,
nitroazobenzoic acid and carboxylic acid are produced.