Ionisation Potential : What are the factors that decide the ionisation potential?
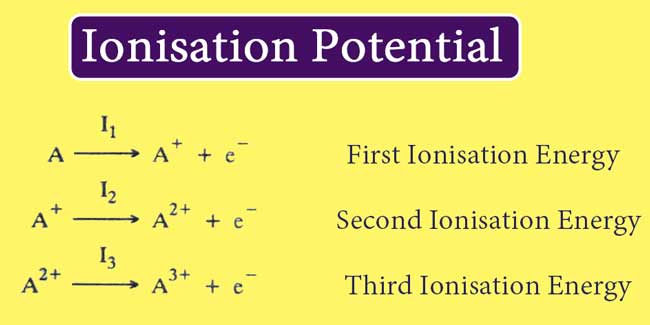
The amount of energy required to remove one electron from an isolated gaseous atom of an element is known as the ionisation energy or ionisation potential of that element.
When only 1 electron is ejected from one atom of an element. Then there is the avidity of energy, it is called the first Ionisation potential of that element.
Similarly, the amount of energy required to exclude the second and third electrons is called second ionisation potential and third ionisation potential, respectively.
Ionisation potential depends on the following factors.
Nuclear charge: The higher the nuclear charge, the greater will be the attraction between the electrons and the nucleus. Hence more energy will be required to separate the electron. Hence Ionisation potential will be high.
Size of the Atom: The larger the size of an atom, the lower the attraction between the outermost electron and the nucleus. Therefore, less energy will be required to separate the electrons. Hence Ionisation potential will be less.
Screening Effect: The electrons of the shells before the last atom act as a screen between the nucleus of the atom and the electron of its external shell.
This effect is called cover effect. Effective charge of the nucleus decreases due to the dielectric effect.
The attraction between the nucleus and the electron of the external shell decreases, the value of the energy required to separate the electron is reduced and the ionisation potential will be less.
The casing effect of d and f-subfamily is less than the casing effect of s and p- subfamily.
Effect of Subshells: s-electrons of a shell are closest to the nucleus and f – electrons are closest to the nucleus. s-electrons are more attracted towards the nucleus and f- electrons are attracted towards the nucleus.
Hence the ionization potential decreases in this order-
s-Electon > p-electron > d-electron > f-electron
Stability of electronic arrangement: If an atom or ion has half or fully filled subcells present, it’s electronic arrangement is considered more stable.
If the initial electronic system is more stable than the ionization potential value will be higher. If the electronic system obtained as a result of ionization is more stable than the value of ionization potential will be lower.
Ionisation Potential in Periodic table
In the periodic table, the ionization potential in the periods and groups changes as follows:
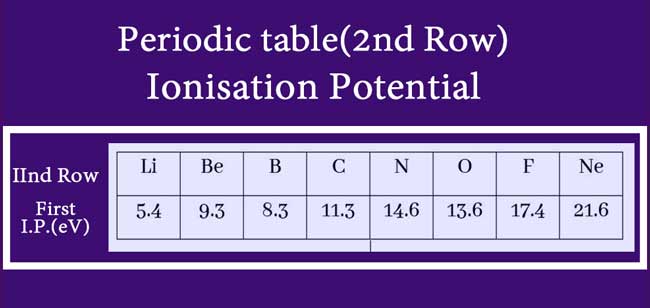
In a period, ionization potential increases from left to left.
The reason for this is that when moving from left to left in the periodic table, the amount of nuclear charge increases and the atomic radius decreases.
As a result, the attraction between the nucleus and external electron increases. The value of the energy required to separate the external electron increases and the ionisation potential increases.
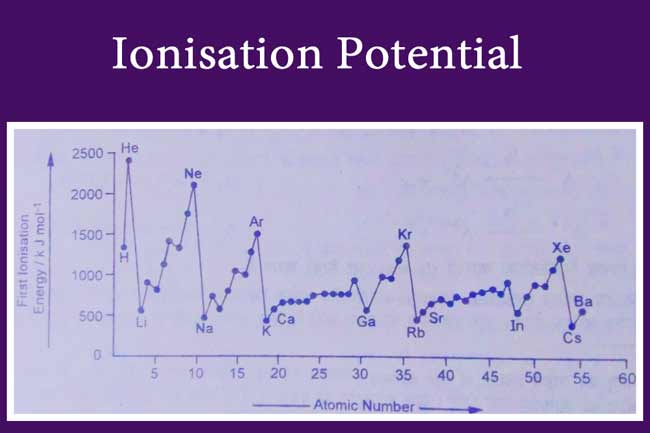
The ionisation potential of an inert gas located in a periodic table is maximum. One reason for this is that the electronic arrangement of inert gases is permanent and more energy will be required to separate external electrons from them.
The atomic number and first ionisation potential of elements from hydrogen to barium are shown on the graph.
Ionization Energy Example
The atomic number and first ionisation potential of elements from hydrogen to barium are shown on the graph.
This graph shows that ionisation potential increases when moving from left to right in a periodic table but ionization potentials of elements of IIIA subclass are lower than ionization potentials of elements in IIA subclass.
Example:
The ionization potential of boron is less than the ionization potential of beryllium. The reason for this is that the external shell of Be has a coupled s -electone while the external shell of B has unpaired p-electrons.
Energy would be required to eject the unpaired p -electron while more energy would be required to eject the paired s -electron.
Similarly, ionization potentials of elements of VIA subclass are lower than ionization potentials of elements of VA subclass.
Example:
The ionization potential of oxygen is less than the ionization potential of nitrogen. The reason for this is that N has three unpaired electrons in its p subshell in its original state.
That is, his p-sub-shell is half full. Whereas in its ionized state there are two unpaired electrons. Hence the electronic state of the original state of N is more stable than the electronic state in its ionized state.
Hence the ionisation potential of N is high. In contrast, the electronic state of O is less stable than the ionized state in the original state. Hence the ionisation potential of O is less.
The ionisation potential of the transition elements is also frequently increased when left to right in a periodic table, but the last electron in the transition elements enters the last-to-the-first dictionary. Therefore, there is not much difference in their ionization potential due to screening effect.
When moving from top to bottom in a subclass, the ionization potential decreases.
The reason for this is that in a subclass moving from top to bottom, the size of the atom increases. As a result the attraction between the nucleus and the external electron is reduced, less energy is required to separate the electron and ionization potential is reduced.
Along with the increase in nuclear charge, the number of electrons in the inner shells also increases. These act as a screen between the nucleus and external electron. Therefore, with the increase in nuclear charge, the casing effect also increases and there is no significant increase in the effective charge of the nucleus.
Therefore, an increase in nuclear charge when moving from top to bottom in a subclass does not have a special effect on ionisation potential, but an increase in the size of an atom has a special effect on ionization potential.
The second ionization potential is greater than the first ionization potential. Similarly, the third ionization potential is greater than the second ionization potential.
Ionization energy –
chemistry
https://www.britannica.com/science/ionization-energy
By separating one electron from an atom, the process of separating one electron from the obtained positive electric charge is relatively difficult.
By separating one electron from an atom, the radius of obtained positive electric charge is small and the attraction between its nucleus and external electron increases.
Hence the second ionization potential is greater than the first ionization potential.
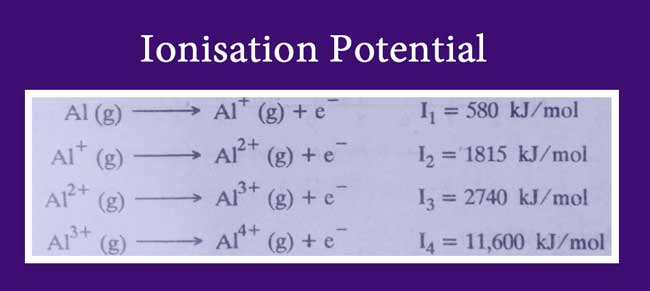
Similarly, the third ionization potential is greater than the second ionization potential if aluminum has a greater difference between the fourth ionization potential and the third ionization potential.
The reason for this is that after the separation of three electrons from the aluminum atom, the number of cells in it decreases. Effective nuclear charge is increased as cell numbers decrease.
And the distance between the nucleus and external electrons decreases. Hence, the attraction and ionization potential increases between the nucleus and electron of the outer shell.
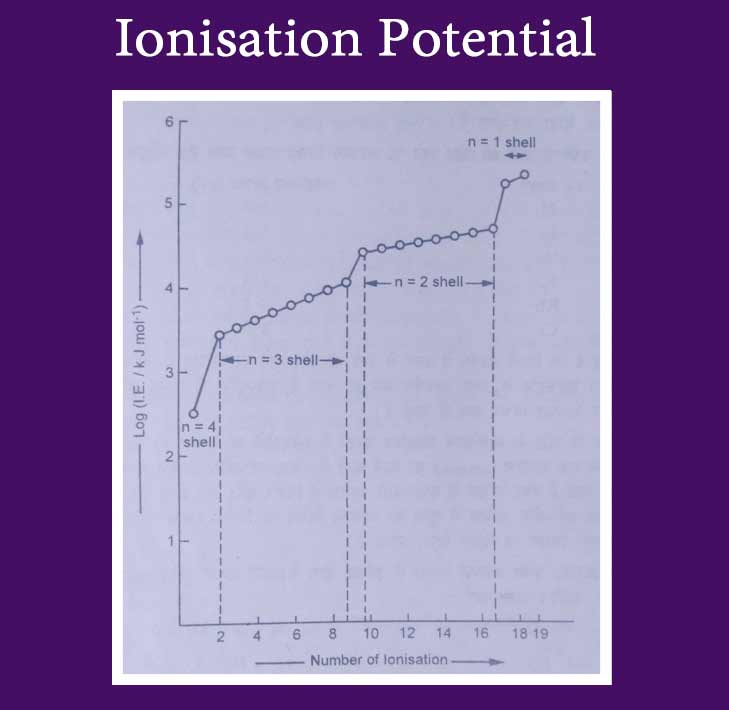
The graph is displayed by drawing a graph between the ionization potential of the potassium atom and the number of ions. In this graph the value of ionization potential increases as the number of ions increases.
The value of ionization potential increases more when the number of ions increases. There is more difference between the first and second ionization potentials.
Similarly, there is a difference between the 9th and 10th ionization potentials and the 17th and 18th ionization potentials. A greater difference in ionisation potential reflects a change in the number of rocks. On this basis, it is known that there is one electron in the external shell (n = 4) of potassium atom, 8 in n = 3, 8 in n = 2 and two in n = 1.