Physical Properties of Iron || What is Iron use for || Iron Deficiency
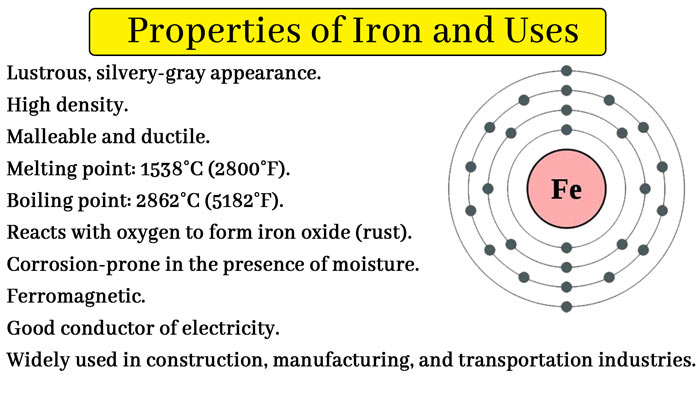
Iron (iron) is a metal element. The iron atomic number is 26. Fe is the chemical formula of iron. Pure iron is white or silver-white with a metallic luster.
It has a melting point of 1538°C and a boiling point of 2750°C. It is soluble in strong and medium-strong acids and insoluble in water. Iron has 0 prices, +2 price, +3 price, +4 price, +5 price and +6 price, of which +2 price and +3 price are more common, +4 price, +5 price, and +6 price are rare.
Iron is widely distributed in life, accounting for 4.75% of the crust content, second only to oxygen, silicon, and aluminum, and ranks fourth in the crust content. Pure iron is a flexible and malleable silver-white metal. It is used to make iron cores for generators and motors. Iron and its compounds are also used to make magnets, drugs, inks, pigments, abrasives, etc. One of the “ferrous metals”.

In addition, the human body also contains iron, and the ferrous ion of +2 is an important component of hemoglobin, which is used for the transportation of oxygen.
The main iron ore used is:
- Fe2O3 (hematite)
- Fe3O4 (magnetite)
- FeCO3 (siderite)
- FeS2 (pyrite)
Physical Properties
Appearance and shape: Pure iron is a metallic crystal with a silver-white metallic luster, and is usually gray to gray-black high-purity iron wire color amorphous fine particles or powder.
It has good ductility, electrical conductivity, and thermal conductivity.
Strong ferromagnetic belongs to the magnetic material.
The specific heat capacity was 460J/(kg·°C).
The sound transmission rate in iron: 5120m/s.
Pure iron is soft, but if it is an alloy of iron with other metals or iron-doped with impurities, usually the melting point decreases and the hardness increases.
Crystal structure: face-centered cubic and body-centered cubic.
The relative atomic mass of iron is 55.85, and the atomic electron arrangement of the ground state is: 1s22s22p63s23p63d64s2.
Chemical Properties
Iron is an indispensable metal in the industrial sector. Iron and a small amount of carbon are made into an alloy – steel, which is not easy to be demagnetized after magnetization. It is an excellent hard magnetic material, and it is also an important industrial material. There are many allotropes of iron.
Iron is a more reactive metal, the metal active sequence table in the front row of hydrogen, the chemical nature of the lively, is a good reducing agent. Iron cannot burn in air, but it can burn violently in oxygen.
Iron is a valence element. The valence of 0 is only reducing, the valence of +6 is oxidizing, and the valences of +2 and +3 are both reducing and oxidizing. In the displacement reaction, it is generally +2 valence, but a few are +3 valence, such as the reaction of ferrous bromide and excess chlorine:
2FeBr2 + 3Cl2 (Excess) = 2FeCl3 + 2Br2
At room temperature, iron does not easily react with non-metallic elements such as oxygen, sulfur, and chlorine in dry air. If there are impurities, it is easy to rust in humid air, it grows in wet air in the presence of acid, alkali or salt solutions.
Rust is faster. At high temperatures, a violent reaction occurs, such as the burning of iron in oxygen to form Fe3O4, and the red hot iron reacts with water vapor to form Fe3O4.
When heated, it can combine with halogen, sulfur, silicon, carbon, phosphorus and so on. In addition to +2 and +3 valence oxides, a complex oxide Fe3O4 is also formed.
Iron easily dissolves in dilute inorganic acids, generates divalent iron salts, and emits hydrogen. When meeting concentrated sulphuric acid or concentrated nitric acid at normal temperature, a layer of oxide protective film is formed on the surface to make the iron “passivate”, so iron products can be used to hold cold concentrated sulfuric acid or cold concentrated nitric acid.
When heated, iron can react with concentrated sulfuric acid or concentrated nitric acid to form a + 3-valent iron salt, and at the same time generate SO2 or NO2.
Chemical reaction
Combustion of iron wire in pure oxygen
1. Iron burns in oxygen
Combustion of iron wire in oxygen:
At high temperatures, iron burns in pure oxygen react violently and radiate from Mars to form Fe3O4. Fe3O4 can be regarded as FeO·Fe2O3. (It should be noted that the reason why iron burns Martian radiation in oxygen is that iron wire usually contains a small amount of carbon, and pure iron combustion will hardly have Martian radiation.) Reaction equation:
3Fe + 2O2 = Fe3O4 (ignite).
2. Iron reacts with the acid
The reaction of iron with non-oxidizing dilute acid (displacement reaction)
Fe + 2H+ = Fe2+ + H2
Generally, iron reacts with dilute sulfuric acid to form ferrous sulfate, and bubbles are generated. In reality, it is more complicated. However, when iron meets cold concentrated sulfuric acid or concentrated nitric acid, it will passivate and form a dense oxide film (main component Fe3O4). Therefore, iron sulfate can be used to ship concentrated sulfuric acid and concentrated nitric acid. The equation is:


When iron reacts with non-oxidizing acid, sulfuric acid, sulfur, copper sulfate solution, etc. it loses two electrons and becomes +2 valence when reacting with nitric acid, it depends on the ratio of the amount of substance and the concentration of nitric acid (the following concentrated represents the concentration, Dilute stands for dilute and excess stands for excess).

3. The reaction of iron with salt
Example: Replacement reaction between iron and copper sulfate solution:

(Principle of wet copper smelting) This experiment shows that iron has more metal mobility than copper.
4. Redox reaction of Fe 3+

(It can react at normal temperature and is used to etch copper plate)

(The reason why Fe 3+ and I – cannot coexist)
Fe + 2Fe3+ = 3Fe2+
(Iron generally shows +2 valence in the replacement)
5. React with halogen
Example: A chemical reaction between iron and chlorine:
2Fe + 3Cl2 = 2FeCl3
Iron and chlorine react violently when ignited, producing a large amount of brown-red smoke (FeCl 3 small particles) that react with Cl 2 and Br 2 to be oxidized to Fe 3+, and react with 12 to be oxidized to Fe 2+. The equation is:
2Fe + 3Br2 = 2FeBr3
(chemical reaction)
Fe + I2 = FeI2
(chemical reaction)
6. Reaction with water should

(High temperature means high temperature) (displacement reaction)
7. React with elemental sulfur
Fe + S= FeS (chemical reaction)
Iron can also combine directly with sulfur, phosphorus, silicon, and carbon. Iron and nitrogen cannot be combined directly, but interact with ammonia to form iron nitride Fe₂N.
8. Other
Divalent iron ions are light green and easily oxidized to trivalent iron ions in alkaline solutions. The color of ferric ions changes from yellow to orange to brown with an increasing degree of hydrolysis. Pure trivalent iron ions are lavender. Both divalent and trivalent iron easily form stable coordination compounds with inorganic or organic ligands.
For example, Phen is phenanthroline, and the coordination number is usually 6. Zero valent iron can also form various carbonyl irons with carbon monoxide, such as Fe(CO)5, Fe2(CO)9, Fe3(CO)12. Iron carbonyl is volatile and vapor is highly toxic. Iron also has +4, +5, +6 valence compounds, but only +6 (FeO4 2- ) in aqueous solution.
Knowledge of iron
Iron deficiency anemia is one of the four major nutritional deficiencies identified by the World Health Organization.
- In the 18th century, Menghini used magnets to adsorb particles in dried blood, noting that the blood contained iron.
- In 1892 Bunge noted that infants and young children were prone to lack of iron.
- In 1928, Mackay first proved that iron deficiency was the cause of anemia in infants and young children in the East End of London. She also thought that providing iron-fortified milk powder could alleviate anemia.
- In 1932, Castle and colleagues confirmed that inorganic iron could be used for hemoglobin synthesis.
Intake
The World Health Organization recommends that the amount of iron (iron food) be 5-9 mg for adult men and 14-28 mg for adult women. Infants to 9 years of age require 10 mg of iron per day, children of 10 to 12 years (children’s food) require 12 mg of iron, young men (male food) of 13 to 18 years require 15 mg of iron, and juvenile (juvenile food) 20 mg for women, 12 mg per day for people over 18 years of age, but 18 mg for adult women (female food). Nursing mothers, pregnant women (pregnant women’s food) is 28 mg.
Recommended daily intake:
Adult intake is 10-15mg; pregnant women need 30mg.
In one month, women lose about twice as much iron as men (men’s food); copper, cobalt, manganese, and vitamin C are needed to absorb iron.