IUPAC Name : How to find the IUPAC name of compounds.
From 1892 to 1958 several other methods of naming organic compounds were published. The scientists presenting them believed that these methods were systematic and sequential, but none of these methods were considered satisfactory for long.
In 1958, the International Union of Pure and Applied Chemists IUPAC introduced a hierarchical method of naming organic compounds. This method is called the IUPAC system or modern system. Names written according to a method of organic compounds are called their IUPAC names.
In this method, the names of organic compounds are written on the basis of IUPAC names of alkanes that contain all the carbon atoms present in the same straight chain. Such alkane is called analog alkane or similar alkane. The following are the atoms of such alkane, structure, common name and IUPAC name.
In this method some rules have been made for writing the names of organic compounds. With the help of these rules, the names of organic compounds can be written and their names can be seen. And by looking at their names, their structures can be written.
Thus, this method has specific relation to the names and structures of organic compounds and this method is a systematic and sequential method.
The major advantage of a method is that it is not necessary to remember the names of all organic compounds, IUPAC name of all compounds can be written only after remembering certain rules and the names of the above alkane.
One drawback of this method is that some of the names given by this method according to this method are so difficult and long that it is inconvenient to use them again and again. For this reason the simple names of many compounds are more popular than their iupac names.
IUPAC Nomenclature (Basic concepts)
Organic Chemistry: Some Basic Principles and Techniques “GOC”
IUPAC name method of simple compounds
IUPAC names for compounds in which only one endowment is present or only one functional group is present are written with the help of the following three rules.
Rule 01
First, select the longest chain of carbon atoms in a given compound. After this, write the name of the corresponding alkane based on the number of carbon atoms present in this series.
Example: CH3CH2CH2COOH has 4 carbon atoms in a series of carbon atoms and the alkane corresponding to this additive is named butane.
Rule 02
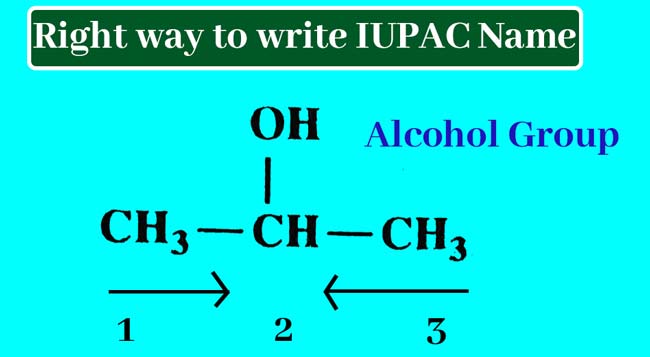
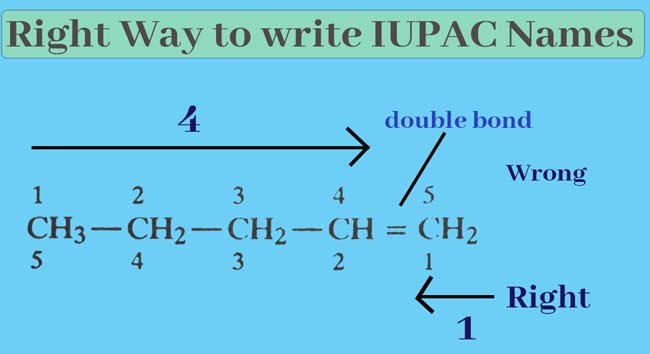
Write the order of the number of carbon atoms in the selected series of carbon atoms. The sequence of numbers starts from the end that gives the lowest number of carbon atoms carrying a lateral chain or functional group.
The numbers of carbon atoms in CH3-CH2-CH2-CH=CH2 can be written from the left or right side.
- IUPAC Name : How to find the IUPAC name of compounds.
- Molarity Formula : What is Molarity and Normality with Solved Examples?
- Glass Material | Composition, Types of Glass, How to make colored glass?
- Ionisation Potential : What are the factors that decide the ionisation potential?
- Fertilizers Chemistry : Types of Fertilizers used in agriculture
Since the sequence of numbers starts from the left, the number of double-bonding atoms comes to 4 and when it starts from the right comes to 1, so it is right to start the sequence of numbers from the right.

Rule 03
The presence of a lateral chain or functional group present in an organic compound is represented as follows
Carbon-carbon double-bonding
If carbon-carbon double-bonding is present in the given compound, remove ‘ane’ from the end of the name of the corresponding alkane and put ‘ene’.
Alkane – ane + ene = Alkene
In this way, putting a hyphen in front of the name obtained, also write down the number of carbon atoms carrying double bond. Thus the IUPAC name of the given additive is obtained.

According to the above rules, the IUPAC name of the first compound above is derived 1 – methene. In this compound only one position of double bond i.e. position number 1 is possible. Hence no additional information is available from prefix 1.
The same information is obtained from the name ethene, which is derived from the name 1 – ethene, so the IUPAC name of this compound is not written as 1 – ethene and only ethene is written. Similarly, in the IUPAC name of some other compounds, there is no benefit of displaying the status of the functional group.
Carbon-Carbon tri-bond
If carbon-carbon tri-bond is present in the given organic compound, then ane is removed from the end of the name of the corresponding alkane and add yne.
Alkane – ane + yne = Alkyne
Thus, putting a hyphen before the name obtained, we also write the number of carbon atoms carrying the tri bond, thus the IUPAC name of the given compound is obtained.

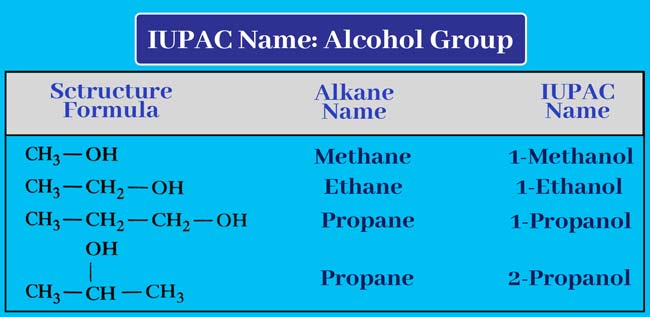
Alcohol Group
If an alcoholic group is present in the given organic compound, then add ol at the end of the name of the corresponding alkane. remove ‘e’ and add ‘ol’.
Alkane – e + ol = Alkanol
Thus by putting a hyphen before the name obtained, we also write the number of carbon atoms that carry the –OH group. Thus the IUPAC name of the given compound is obtained.
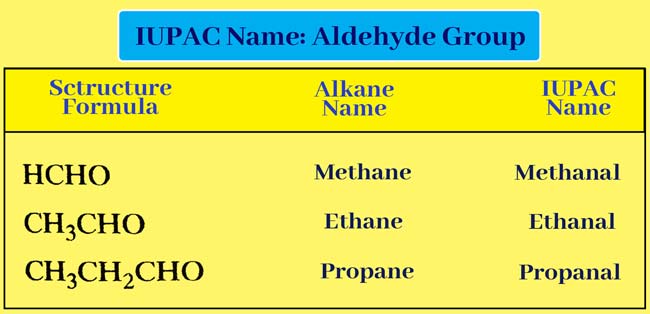
Aldehyde Group
If aldehyde group is present in a given organic compound, then ‘e’ is removed from the end of the corresponding alkane name and added to ‘al’.
Alkane – e + al = Alkanal
Thus the IUPAC name of the given compound is obtained.
Ketone Group
If ketone group is present in the given organic compound, then ‘e’ is removed from the end of the corresponding alkane name and add ‘one’.
Alkane – e + one = Alkanone
In this way, putting a hyphen(-) before the name obtained, we also write the number of carbon atoms related to the ketone group. Thus the IUPAC name of the given compound is obtained.

Carboxyl Group
When carboxyl group (-COOH) is present in an organic compound, then ‘e’ is removed from the end of the corresponding alkane name and add ‘oic acid’.
Alkane – e + oic acid = Alkanoic Acid
Thus the IUPAC name of the given compound is obtained.

Carboxylic acid chloride group
If a given organic compound contains carboxylic acid chloride group (-COCl), then ‘e’ is removed from the end of the corresponding alkane name and add ‘oyl chloride’.
Alkane – e + oyl chloride = Alkanoyl chloride
Thus the IUPAC name of the given compound is obtained.

Carboxylic acid amide group
If carboxylic acid amide group (-CONH2) is present in the given organic compound, remove ‘e’ from the end of the corresponding alkane name and add ‘amide’.
Alkane – e + amide = Alkanamide
Thus the IUPAC name of the given compound is obtained.

Carboxylic acid Anhydride group
If a given organic compound contains a carboxylic acid anhydride group (-CO-O-CO-) It means it is an acid anhydride, then its IUPAC name is written based on the IPUAC name of the corresponding acid. To write the name of acid anhydride, remove the ‘acid’ from the end of the name of the respective acid and write it as ‘anhydride’.
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Is methane gas harmful to humans?|| Why Methane is a good fuel
- DDT full Form || What was DDT used for? || Is DDT harmful to humans?
Example: (CH3CO)2O is an acid anhydride. It is an anhydride of CH3COOH. The IUPAC name of CH3COOH is ethanoic acid, so the IUPAC name of (CH3CO)2O is ethanoic anhydride.
Carboxylic ester group
If a given compound contains carboxylic ester group (-COOR) therefore it is carboxylic ester then its IUPAC name is written on the basis of the IUPAC name of the corresponding acid.
To write the name of the ester, remove the ‘Oic acid’ from the end of the name of the respective acid and write the ‘Oate’ and thus write the name of the alkane group present in the ester group before the name obtained.
Example: CH3COOC2H5 is an ester. The acid associated with this ester is CH3COOH. The IUPAC name of CH3COOH is Ethanoic Acid. The alkane group of the ester group
is ethile. Hence the IUPAC name of CH3COOC2H5 is ethyl ethanoate.
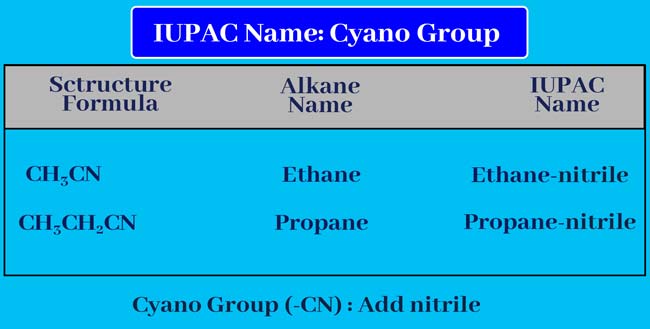
Cyano Group
if cyano (-CN) group is present in the given organic compound. So add nitrile to the end of the name of the corresponding alkane.
Alkane + nitrile = Alkanenitrile
Thus, the given organic compound gets its IUPAC name.
Amino Group
If a given organic compound contains a primary amine (-NH2) group. So remove e from the end of the corresponding alkane name and add amine.
Alkane – e + amine = Alkanamine
In this way, putting a hyphen before the name obtained, we also write the number of carbon atoms carrying the amine group. Thus the IUPAC name of the given compound is obtained.

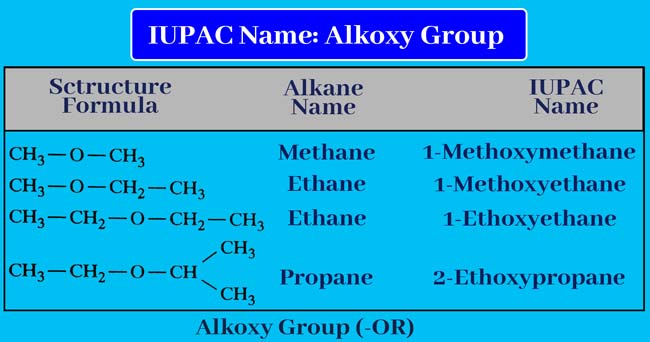
Alkoxy group
If alkoxy group (-OR) is present in the given organic compound. It is means it is an ether, then write the name of this group before the name of the corresponding alkane. Write the name of the corresponding alkane and the name of the alkoxy group together or put a hyphen between them.
Following are some alkoxy group names

Thus by writing a hyphen before the name obtained, we also write the
number of carbon atoms carrying the alkoxy group. It is not necessary to
write this number if only one position of a carbon atom carrying alkoxy
group is possible. Thus the IUPAC name of the given compound is
obtained.
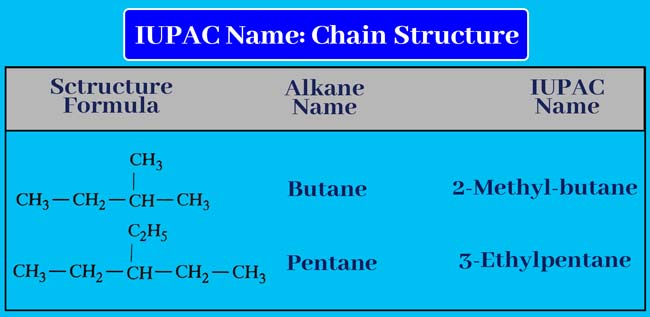
Carcass chain
If a carcass chain is present in a given organic compound. Write the name of that series (alkane group) before the name of the corresponding alkane. Write the name of the corresponding alkane and the name of the alkyl group together or put a hyphen between them.
Thus, by putting a hyphen before the name obtained, we also write down the number of carbon atoms carrying the lateral chain. Thus the IUPAC name of the given compound is obtained.
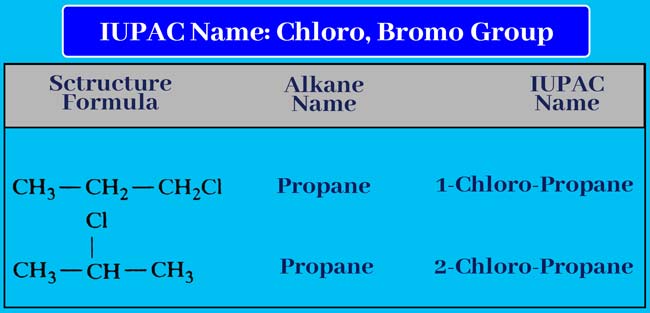
Chloro bromo or Iodo group
If a given compound contains chloro, bromo or Iodo group, then the IUPAC name of those compounds is written like the rule above. The name of the group is written before the name of the corresponding alkane and before that the status of the group is displayed.
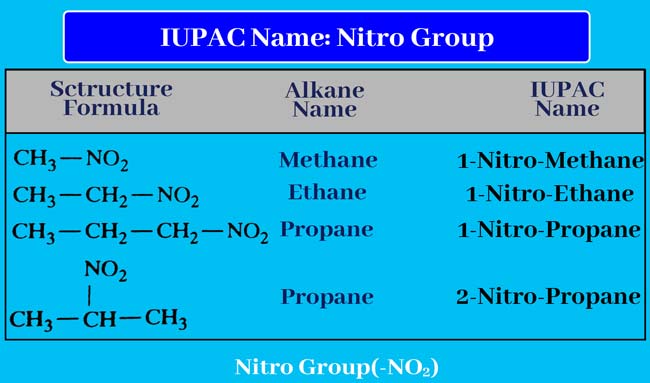
Nitro group
If nitro (-NO2) group is present in a given compound, then the IUPAC name of that compound is also written like the above rule. Write the name of the group before the name of the corresponding alkane and display the status number of the group by writing it.
IUPAC Nomenclature Rules
Note 01: Simple Compounds When writing the IUPAC names of organic compounds, -COOH, -COCl, -CONH2, -COOR, -CHO, -CO and -CN group, the carbon atoms of carbon-carbon bond and carbon-carbon bond are always considered part of the chosen chain.
Note 02: In a simple organic compound, the position number of the carbon atom belonging to the –COOH, -COCl, -CONH2, -COOR, -CHO and -CN group is always 1.
Therefore, there is no benefit in writing the position number of carbon atoms belonging to the group in conditions. Therefore, in these cases, the position number of the carbon atom belonging to the group is not written, in some other cases also, the position number of the carbon atom belonging to the group is not written.
IUPAC Nomenclature Practice
Following are some examples of writing IUPAC names of simple organic compounds.
Example 01: Find the IUPAC name of following chemical structure:
CH3 – CH2 – C = C – CH3
Answer: This compound contains only one series of carbon atoms which has 5 carbon atoms. Hence the corresponding alkane name of this compound is penten.
The order of the number of carbon atoms in the series of carbon atoms, starting from the left, the number of tri-bonding atoms comes to 3 and the starting from the right comes to 2. Therefore, it is right to start the sequence of numbers from the right.
When carbon-carbon tri-bond is present, remove ‘ane’ from the end of the name of the corresponding alkane and add ‘ion’.
pentane – ane + yne = pentyne
In this way, IUPAC name of the compound is obtained by writing the number of carbon atoms carrying tri bond before putting a hyphen before the name obtained. Hence the IUPAC name of this compound is 2 – Pentyne.
Example 01: Find the IUPAC name of following chemical structure:
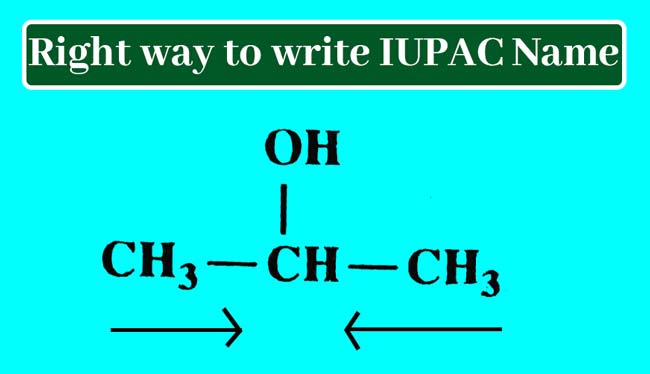
Answer: This compound has only one series of carbon atoms which has three carbon atoms. Hence the corresponding alkane name of this compound is propen.
Starting the sequence of the number of carbon atoms in a series of carbon atoms, the number of carbon atoms carrying the –OH group is 2.
The –OH group present in this compound is the alcohol group. To display the alcohol group present, remove the en at the end of the corresponding alkane name and add ol.
propen – en + ol = propanol
Thus, IUPAC name of this compound is obtained by writing the number of carbon atoms that carry the alcohol group by applying a hyphen before the name obtained. Hence the IUPAC name of this compound is 2-propanol.