What do you mean by the Law of Mass Action?
Law of Mass Action
Example:
AB + C → AC + B
In the above reaction, the affinity of A is greater than that of B towards C. Hence, AB decomposes first and then combines with A to form AC and separates B. On the basis of chemical affinity, elements are categorized in an order and affinity tables are drawn.
The idea of chemical affinity could not be recognized because reversible reactions cannot be explained clearly.
- Daily use Chemical Compounds and Their Properties
- Hard Water and Soft Water : Permutit and Anion Exchange Resins
- Properties of Water :- Purification, Sources and Uses
- Hydrogen Chloride : Preparation, Properties and Uses
- Washing Soda and Baking Soda : Difference and Properties
- Electric Cell, Terminal Potential difference, EMF and Internal Resistance
- Electrochemistry : Electrolytic cell, Mechanism and Cell Structure
- Dalton's law of partial pressure || Numeric Example
Berthollet suggested that the direction of a chemical reaction is influenced by the nature of the substance as well as its relative mass, in an article published in 1799 after the country in Egypt.
Based on this, he explained the formation of sodium carbonate found on the banks of some lakes of Egypt. Generally, sodium carbonate and calcium chloride react by intermingling to give calcium carbonate precipitate.
Na2CO3 + CaCl2 → CaCO3 + 2NaCl
The amount of salt in Egyptian lakes is very high, which causes the direction of the reaction to reverse and the salt reacts with the calcium carbonate present at the edges to form sodium carbonate, which is collected.
2NaCl + CaCO3 → Na2CO3 + CaCl2
Mass Action Definition
At constant temperature, the rate at which a substance reacts is proportional to its active mass and the rate of a chemical reaction is proportional to the product of the active masses of the reactants.
The mass action of a substance is expressed in parentheses by writing the formula or symbol of the substance.
Example:
Therefore, excessive mass of a substance replenishes weak chemical affinity and changes the direction of reaction. These ideas given by Berthollet have been confirmed by many experiments.
In 1867, two Norway chemists, Gulberg and Wage, studied the effect of concentrations of reactants on the velocity of many reactions, and formulated a law based on what is called the law of mass compensation.
The mass action of a substance is expressed in
parentheses by writing the formula or symbol of the substance.
Example:
The active mass of Cl2 is expressed by [Cl2].
Let the equation of a reaction be as follows –
A + B → Products
According to the law of substance proportional action –
Reaction velocity ∞ [A] x [B]
Let the equation of another reaction be as follows –
2A + B → Products
The above equation can also be written as follows.
A + A + B → Products
According to the law of substance proportional action –
Reaction velocity ∞ [A] x [A] x [B]
Reaction velocity ∞ [A]2 x [B]
Similarly for the following reaction
aA + bB → Products
Reaction velocity ∞ [A]a x [B]b
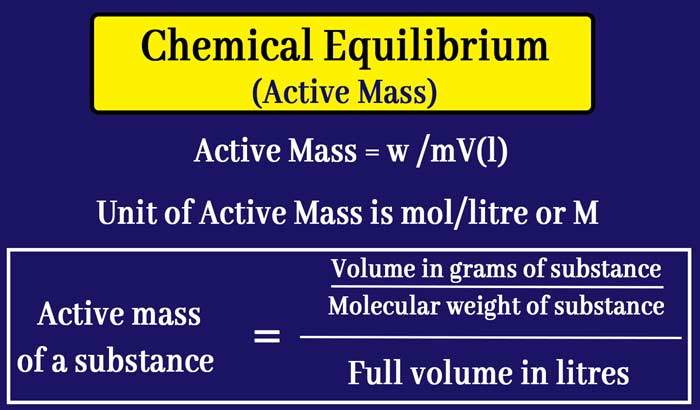
The active mass of a substance usually refers to its molar concentration. The molar concentration of a substance is equal to the number of moles in that substance present in a 1 litre solution. Therefore
Active mass of a substance = molar concentration of a substance
= Number of moles of substance / absolute volume in litres
= Volume in grams of substance / Molecular weight of substance / Full volume in litres
The unit of active mass is mole/liter or M.
Mass Action Important Notes
- In gaseous reactions, the active masses of gases are equal to their molar concentrations.
- In dilute solutions, the active masses of solute are equal to their molar concentrations.
- In concentrated solutions, the active masses of solute are less than their molar concentrations. The reason for this is that in concentrated solutions, the molecules of solute do not separate and remain as groups or aggregates.
- If a solid or liquid is present in the hetrogeneous phase, ie, it is not soluble in the phase in which the reaction takes place, its molar concentration is constant and its active mass is assumed to be 1.
- If gas is present in a heterogeneous phase, its active mass at 1 atmospheric pressure is considered to be 1. x is assumed to be its active mass x at atmospheric pressure.
- In dilute solutions, the molar concentration of water is 55.55 mole / litre and remains constant.
500 ml of a solution contains 15 grams of acetic acid and 8 grams of methanol. What will be its active mass?
Answer: molecular weight of acetic acid (CH3COOH) = 2 x 12 + 4 x 1 + 2 x 16 = 60
Active mass of acetic acid (CH3COOH) = weight of (CH3COOH) in grams / atomic mass of (CH3COOH) / full volume in litres
= 15/60/½
= 0.5 mole/litre
Molecule of Methanol (CH3OH) = 12 + 4 x 1 + 16 = 32
Active mass of methanol (CH3OH) = weight of (CH3OH) in grams / atomic mass of (CH3OH) / full volume in litres
= 8/32 / ½
= 0.5 mole / litre