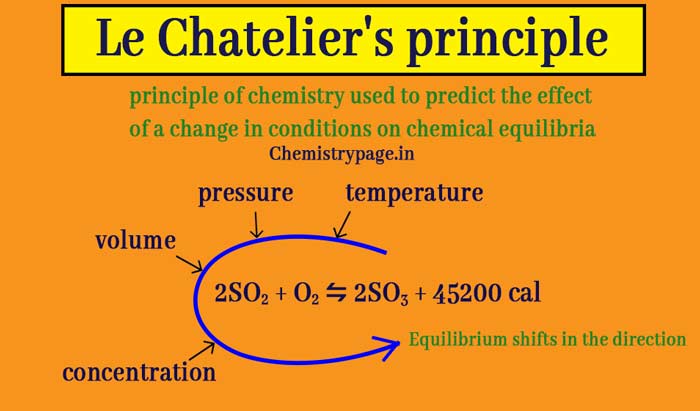
What is Le Chatelier’s principle in chemistry? Temperature and pressure effect
According to the law of mass action, the quantitative effects of temperature, pressure, concentration, volume and other factors on the equilibrium of a chemical reaction can be studied. For the qualitative study of these factors on equilibrium, French chemist Henry Louis Le Chatelier introduced a generalized rule in 1884 called le chatelier’s Principle.
According to this rule –
If a system in equilibrium is subjected to a charge of concentration, pressure, temperature, volume etc., the equilibrium shifts in the direction that tends to undo the effect of the change.
Le Chatelier’s principle effect
According to this rule, the effect of change in temperature, pressure, concentration and volume on the equilibrium is as follows –
Effect of heat change
Two mutually opposite reactions occur simultaneously at equilibrium. One reaction is exothermic and the other reaction is endothermic. Increasing the temperature will increase the velocity of the reaction in which the effect of increasing the heat is overcome, that is, in which the heat is absorbed.
Therefore, the velocity of exothermic reaction increases with increasing temperature. In other words, by increasing the temperature, the equilibrium migrates towards the exothermic reaction.
Effect of concentration change
When an element is added from outside to the equilibrium, the equilibrium will be displaced further, which decreases the concentration of that component.
Therefore, on increasing the concentration of the reactants, the equilibrium is displaced in the forward direction and on increasing the concentration of the products, the system is displaced in the opposite direction.
Effect of pressure changes
According to Le chatelier’s Principle, by increasing the pressure, the equilibrium will be displaced towards which the pressure is reduced. In a reaction in which the number of molecules of gaseous substances is small, the pressure is also low (P ∞ n).
Therefore, by increasing the pressure, the equilibrium is displaced towards which there is a decrease in the number of molecules of gaseous substances. If the number of molecules of gaseous substances on both sides is the same then the pressure change has no effect.
Effect of volume change
If the volume increases then the pressure decreases (P ∞ 1/V). Therefore, by increasing the volume, the equilibrium is displaced towards which the number of molecules of gaseous substances increases.
Physical Law Example
Following are the major examples of the application of le chatelier’s Principle on physical equations.
Effect of heat on solubility of solids
Most solids are dissolved in water, heat is absorbed and the heat decreases. Example:
On dissolving potassium nitrate (KNO3) in water, the following equilibrium is formed in a saturated solution of solids KNO3 and KNO3 –
KNO3(s) + aq ⇋ KNO3(aq) – 8.34 kcal
If the temperature of this system is increased, the equilibrium will be displaced to the side where the effect of heat increase is destroyed, that is, the heat is reduced. In this equilibrium, the heat is reduced in the forward direction.
Therefore, equilibrium will be displaced in forward direction. Therefore, solubility of KNO3 increases with increasing temperature.
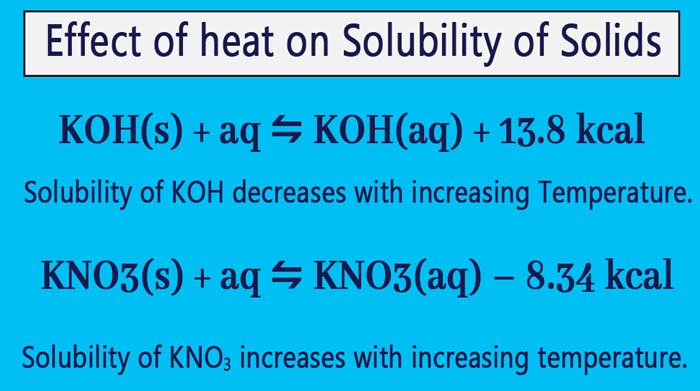
In contrast, some solids produce heat when dissolved in water.
Example:
KOH(s) + aq ⇋ KOH(aq) + 13.8 kcal
By increasing the temperature, the equilibrium will migrate to the side where the heat is reduced. In this equilibrium, the temperature increases in the forward direction and the temperature decreases in the opposite direction.
Therefore, by increasing the temperature, the equilibrium will be displaced in the opposite direction. Therefore, solubility of KOH decreases with increasing temperature. In this way, heat is produced when NaOH, Ca(OH)2 and Ca(CH3COO)2 are dissolved in water and their solubility decreases in water by increasing the temperature.
Effect of pressure on solubility of gas
When the gas dissolves in a liquid, the following equilibrium is established.
Gas ⇋ Liquid
By increasing the pressure, the equilibrium will be displaced on the side where the effect of pressure increase is destroyed, that is, the pressure decreases. In the above equilibrium, the pressure decreases in the forward direction.
Therefore, by increasing the pressure, the equilibrium will be displaced in forward direction. In other words, the solubility of gases increases in fluid upon increasing pressure.
 Melting of ice
Melting of ice
The following equilibrium is established in ice and water at the melting point of ice (0°C) –
Ice ⇋ water – 80 cal/gram
Heat is exploited when the ice is dissolved and the volume is reduced. Therefore, this equilibrium has the effect of change of heat and pressure.
Effect of heat: Due to the effect of heat, equilibrium is displaced towards which heat is absorbed. Therefore, due to the effect of heat, the equilibrium will be displaced in forward direction, that is, the melting of ice will increase.
- What is Le Chatelier’s principle in chemistry? Temperature and pressure effect
- Definition of the Rate Constant and Equilibrium Constant
- What do you mean by the Law of Mass Action?
- What is acetone used for? | Preparation, Properties, Uses, and Tests
- What is Acetaldehyde made of? | Properties, uses, and Tests
Pressure effect: Volume is reduced in the forward direction of the above equilibrium. Increasing the pressure on a system reduces its volume.
Therefore, increase in pressure will displace the equilibrium in the direction where the effect of pressure increase is destroyed, ie the volume is reduced. Hence the equilibrium will be displaced in the forward direction. Therefore, increase in pressure helps in melting of ice.
Vaporisation of water
Heat is exploited in the evaporation of water and the volume increases.
The effect of change in heat and pressure on this equilibrium can be known on the basis of le chatelier’s principle–
Water ⇋ water vapour – 540 cal/gram
Effect of heat: Due to the increase of heat, equilibrium is displaced towards which the heat is absorbed. Therefore, the speed of evaporation of water also increases with the increase of heat.
Pressure effect: There is a decrease in pressure in the opposite direction of the above equilibrium. Therefore, due to the increase in pressure, the equilibrium will be displaced in the opposite direction, that is, the evaporation of water will decrease. Therefore increase in pressure increases the boiling point of water. Pressure cooker increases the boiling point of water when cooking lentils, more heat is available to melt the lentils and the lentils cook quickly.
Le chatelier principle on the chemical equation Construction of hydrogen iodide
The reaction of hydrogen and iodine to form hydrogen iodide is exothermic and there is no change in the number of molecules in it.
H2(g) + I2(g) ⇋ 2HI(g) + Q cal
Effect of concentration change: If the concentration of H2(g) or I2(g) or both these substances are increased on the equilibrium, then the system will be displaced in forward direction to destroy the effect of this growth. Similarly, by increasing the concentration of HI(g), the equilibrium will migrate in the opposite direction.
Effect of heat rise: When the temperature increases, the equilibrium is displaced towards which the effect of this increase is dissipated i.e. there is a decrease in heat or heat is absorbed. Since the forward reaction is exothermic, the equilibrium will migrate in the direction of the opposite reaction upon increasing the temperature.
Effect of pressure increase: On increasing the pressure, the equilibrium is displaced towards which there is a decrease in pressure ie there is a decrease in the number of molecules (P ∞ n). The above reaction has the same number of molecules on both sides, so the pressure change has no effect on equilibrium.
Synthesis of nitric oxide
The reaction of synthesis of nitric oxide by the combination of nitrogen and oxygen is endothermic and there is no change in the number of molecules in it.
N2(g) + O2(g) ⇋ 2NO(g) – 43.2 kcal
According to le chatelier’s principle, the effect of concentration, temperature and pressure changes on the equilibrium of this reaction is as follows –
- Synthesis is increased by increasing the concentration of the reactants.
- Synthesis will be higher at higher temperatures.
- Pressure change has no effect.

The above reaction is used in barkland and id’s method of making nitric acid. In this method, the above reaction is carried out at around 3500°C temperature.
Dispersion of phosphorus Pentachloride
This reaction is endothermic and the result is an increase in the number of molecules.
PCl5(g) ⇋ PCl3(g) + Cl2(g) – Q cal
According to le chatelier’s principle-
Effect of concentration change: Increasing the concentration of PCl5 on equilibrium will increase the isolation of PCl5. Addition of PCl3 or Cl2 will reduce the isolation of PCl5.
Effect of heat conversion: The reaction of PCl5 dissociation is endothermic. By increasing the temperature, the equilibrium is displaced to the side where the heat is absorbed. Therefore, increasing the temperature increases the dissolution of PCl5.
Effect of pressure change: On increasing the pressure, the equilibrium is displaced towards which there is a decrease in pressure ie there is a decrease in the number of molecules(P ∞ n). In the above equilibrium there is a decrease in the number of molecules in the direction of the opposite reaction. Therefore, by increasing the pressure, the above equilibrium will be displaced in the direction of the opposite reaction, that is, the amount of dissociation of PCl5 will be reduced.
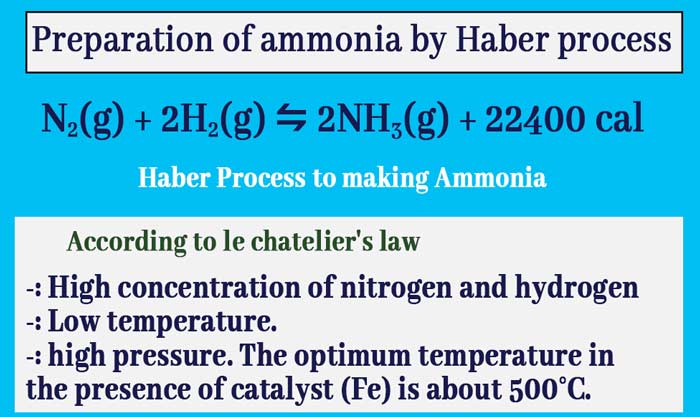
Preparation of ammonia by Haber process
The reaction of nitrogen and hydrogen to produce ammonia is exothermic and there is a decrease in the number of molecules.
N2(g) + 2H2(g) ⇋ 2NH3(g) + 22400 cal
According to le chatelier’s principle:
Effect of concentration change: By increasing the concentration of nitrogen or hydrogen, the equilibrium will be displaced towards which the effect of change is destroyed, that is, the concentration of these substances is reduced.
Therefore, by increasing the concentration of nitrogen or hydrogen, the equilibrium is displaced in forward direction and the amount of ammonia formation increases.
Effect of heat change: By increasing the temperature, the equilibrium is displaced towards which the heat is absorbed. The above reaction is exothermic.
Therefore, by increasing the temperature, the equilibrium is displaced in the opposite reaction. Hence high temperature does not help in overproduction of ammonia. Less heat helps in the formation of more ammonia.
Effect of pressure change: By increasing the pressure, the equilibrium is displaced towards which there is a decrease in pressure. That is, there is a decrease in the number of molecules.
The number of molecules decreases in the forward direction of the above reaction. Therefore, pressure increase helps in more production of ammonia. About 200 atmospheric pressure is kept in the industrial manufacture of ammonia by the Heber method.
It is clear from the above discussion that the optimal conditions for the maximization of ammonia in the reaction of synthesis of ammonia by the combination of nitrogen and hydrogen are as follows.
- i-: high concentration of nitrogen and hydrogen,
- ii-: low temperature,
- iii-: high pressure. The optimum temperature in the presence of catalyst (Fe) is about 500°C.
Preparation of sulfur trioxide
The reaction for the formation of sulfur trioxide (SO3) by the combination of sulfur dioxide (SO2) and oxygen(O2) is exothermic and there is a decrease in the number of molecules.
2SO2 + O2 ⇋ 2SO3 + 45200 cal
According to le chatelier’s principle:
- The increase in concentration of the reactants helps in the formation of more SO3.
- Low heat is helpful in the manufacture of SO3.
- High pressure is helpful in the manufacture of SO3.

The above reaction is used in the lead chamber process and contact process of manufacturing sulfuric acid. Nitrogen oxide in the lead chamber process and platinum(Pt) or vanadium pentoxide(V2O5) act as catalysts in the contact process. The optimum temperature for activation of the catalyst in the contact process is about 450°C.