Method of Preparation of Microcosmic Salt
Microcosmic means man’s. Human urine contains this salt. Therefore, it is called Microcosmic Salt. Its other name is sodium Ammonium Hydrogen Phosphate( (NH4)NaHPO4).

Preparation Of Microcosmic Salt
It is made up of two levels.
First Method:
Disodium phosphate and ammonia chloride are mixed with crystals of micros on mixing and cooling their hot solutions according to equal amounts of their gram weight.
N\a2HPO4 + NH4Cl + 4H2O → Na(NH4)HPO4.4H2O + NaCl
Second Method:
Crystals of microcosmic salts are obtained after cooling by mixing 5 parts of disodium phosphate and two parts of ammonia sulfate by making a hot solution.
Physical Properties:
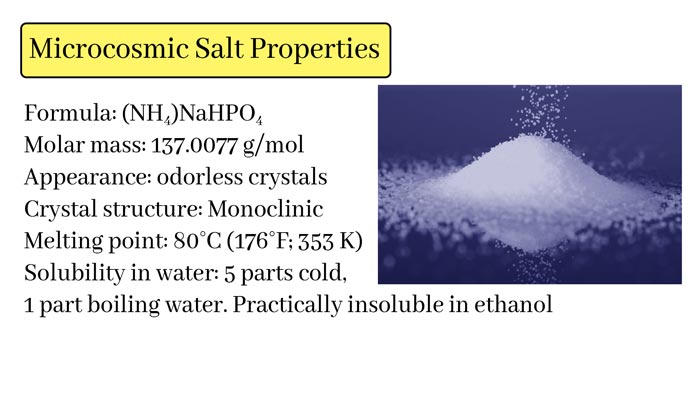
Chemical Formula: (NH₄)NaHPO₄·4H₂O
Appearance: Colorless or white crystals
Solubility: Soluble in water
Acid-Base Properties: Amphiprotic, meaning it can act as
both an acid and a base in solution
Flame Test: Used in flame tests to identify metal ions based
on characteristic colored flames
Analytical Chemistry: Utilized in qualitative inorganic
analysis to separate and identify cations (metal ions)
Applications: Widely used in laboratory experiments and
demonstrations, particularly in educational settings
Specialty Glass and Ceramics: Used in the production of
certain types of glasses and ceramics
Stability: Relatively stable under normal conditions
Storage: Typically stored in dry conditions to prevent moisture absorption and caking
Chemical Properties:
Effects of heat: On heating microcosmic salt, ammonia gas is released and sodium meta-phosphate is formed, which is transparent.
Na(NH4)HPO4.4H2O → NH3 + 5H2O + NaPO3
Sodium forms colored orthophosphates by coinciding with metal oxidation at high temperatures. Due to this property, it is used in qualitative analysis to test metallic roots.
CuO + NaPO3 → CuNaPO4
Similarly, other types of the bead are made. Whose colors are of different types. These colors can be identified to ashes.
Uses of Microcosmic Salt
In qualitative analysis, microcosmic salts are used for dry tests of various asymptomatic roots. This test is called the microcosmic salt bead test.
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
- Why Carbon Cycle is Important || How it Works
- Aldehydes, Ketones and Carboxylic Acids
- Alcohols, Phenols and Ethers
Microcosmic salt, also known as ammonium sodium hydrogen
phosphate, has the chemical formula (NH₄)NaHPO₄·4H₂O. Here are some properties
and uses of microcosmic salt:
Appearance: Microcosmic salt typically appears as
colorless or white crystals.
Solubility: It is soluble in water.
Acid-Base Properties: Microcosmic salt is
amphiprotic, meaning it can act as both an acid and a base. In solution, it can
undergo reactions that demonstrate properties of both acidic and basic
substances.
Flame Test: One of the most common uses of
microcosmic salt is in flame tests for identifying metal ions. When a small
amount of a metal salt is mixed with microcosmic salt and heated in a flame,
characteristic colored flames are produced, allowing for the identification of
the metal ion present.
Analytical Chemistry: Microcosmic salt is used in
qualitative inorganic analysis to separate and identify certain cations (metal
ions) based on the colors of their flame test.
Glass and Ceramics: It is also used in the production
of specialty glasses and ceramics.
Laboratory Reagent: Microcosmic salt is commonly used
as a reagent in laboratory experiments and demonstrations, particularly in
educational settings.
Microcosmic salt plays a significant role in analytical
chemistry, particularly in qualitative analysis, where it is valued for its
ability to form characteristic colored compounds with metal ions.