Neutralisation Reaction Examples: Neutralisation and heat of Neutralization
Acid and base work together to make salts and water. This reaction is called neutralization.
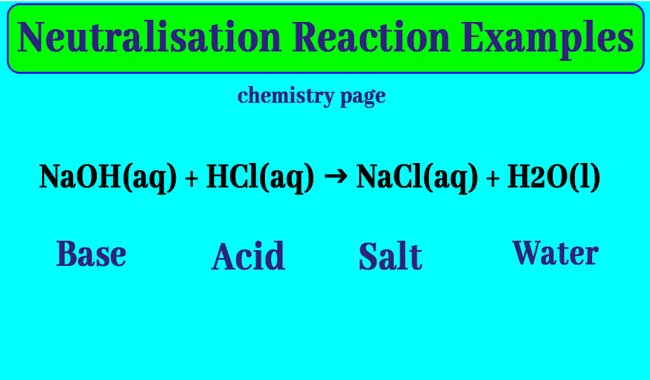
Example:
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
According to ionic theory, the above reaction can also be written as follows.
Na+(aq) + OH–(aq) + H+(aq) + Cl–(aq) → Na+(aq) + Cl–(aq) + H2O(l)
H+(aq) + OH–(aq) → H2O(l)
According to ionic theory, the reaction in which H+ ion and OH– ion form water by interacting with each other is called neutralization. According to the modern concept, the reaction of acid and base is called neutralization.
Heat absorbed in the neutral neutralization of dilute solutions of a gram-Equivalent weight acid and a gram-Equivalent weight base is called heat of neutralization.
Strong acid and strong base dissolve almost completely in their ions in solution. The neutralization heat of strong acid and strong base is actually the reaction heat of the reaction of H+ and OH– to H2O. Hence the value of heat of neutralization of strong acid and strong base is constant.
Heat is emitted in the neutralization reaction. In neutralization reaction of strong acid and strong base, 13.7 kcal of heat is emitted. Hence heat of neutralization of strong acid and strong base is 13.7 Kcal.
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) + 13.7kcal
HNO3(aq) + KOH(aq) → KNO3(aq) + H2O(l) + 13.7kcal
The numerical value of strong acid and strong base heat of neutralisation is less than 13 .7 kcal. Less than 13 kcal of heat is produced in this reaction. The reason for this is that ionization of weak acids and weak bases is not complete.
Initially, H+ derived from acid and OH– ion obtained from base produce 13.7 kcal heat. Some of this heat is used to complete ionization of weak acid and weak base. Therefore, the value of free heat is less than 13.7 kcal and the numerical value of heat of neutralization is less than 13.7 kcal.
Example:
CH3COOH(aq) + NaOH(aq) → CH3COONa(aq) + H2O(l) + 13.3 kcal
HCOOH(aq) + NH4OH(aq) → HCOONH4(aq) + H2O(l) + 11.9kcal
Salts
Acid and base reaction together to make salt and water.
Example:
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)

According to the ionic theory of arhinius, in addition to the neutralization reaction of acid and base, other substances formed in addition to water are called salts.
According to modern ideas, an ionic solid obtained from neutralization reaction of an acid and base is called salts.
Example:
NH3 + HCl → NH4Cl
On dissolving the salts in water, it dissolves in its ions. Cations are called basic radicals and anions are acidic radicals.
Example:
NaCl(aq) ⇌ Na+(aq) + Cl–(aq)
Classification of salts
There are six types of salts –
Normal Salts
Simple salts that do not contain substitutable H+ or OH– are called normal salts.
Example – NaCl and KNO3 are normal salts.
Acidic Salts
Acidic Salts: The salts that contain substitutable H+ are called acidic salts.
NaHSO4 and NaHCO3 are acidic salts.
Basic Salts
Those salts which contain substitutable OH– are called basic salts.
Example: Mg(OH)Cl ZnCl, Pb(OH)NO3 and Zn(OH)Cl is basic salts.
Mixed Salts
Those salts which contain more than one type of cation or anion are called mixed salts. NaKSO4 and Ca(OCl)Cl are mixed salts.
Double Salts
The salts obtained from the evaporation of aqueous solution of two simple salts are called double salts. The ratio of normal salts in double salts is fixed and they exist only in solid state. In aqueous solutions, they dissociate into ions of normal salts.
Example: potash alum[K2SO4.Al2(SO4)3.24H2O] and mohr salt[FeSO4.(NH4)2SO4.6H2O] are double Salts.
Complex Salts
The salts in which ion or neutral molecules form a co-connective bond with a metallic cation are called complex salts.
Example:
K4[Fe(CN)6] and [Cu(NH3)4]Cl2 are complex salts.
K4[Fe(CN)6] has a sub-valence bond between Fe2+ and CN– and [Cu(NH3)4]Cl2 has a sub-valence bond between Cu2+ and NH3.
Complex ions are obtained by dissolving complex salts in water. Complex ions in solutions do not dissociate in their components or have very small amounts of ionization.
K4Fe(CN)6(aq) ⇌ 4K+ + [Fe(CN)6]4-
Salt Hydrolysis
Decomposition of a compound by water is called hydrolysis. The solution obtained after hydrolysis of a salt is neutral, basic and acidic.
Example:
Aqueous solutions of NH4Cl and CuSO4 are acidic, aqueous solutions of Na2CO3 and CH3COONa are basic. And the aqueous solutions of NaCl and KNO3 are neutral. Reaction in which a salt is reacted with water to form an acidic, basic or neutral solution. This is called hydrolysis of salt.
- Sulfuric Acid : Chemical Properties, Uses and Structure
- Rigid Body: Moment of Force or Torque formula
- Preparation of Sulphuric Acid by Contact process with Reaction
- Preparation of Sulfuric Acid by Lead Chamber Process
- Ozone: Tests, Uses and Ozone Structure
- What is Ozone Gas in the air? Ozone Gas Properties.
- Chemical Properties of Hydrogen Peroxide
- What is Deuterium or Heavy Water? How to make Deuterium?
The ionization of water is like this.
H2O ⇌ H+ + OH–
It is neutral due to the same amount of H+ and OH– ion in pure water. If there is more H+ ion in the solution when salt is dissolved in water, then that solution will be acidic and if OH– ion is more, then this solution will be basic.
To simplify the study of the hydrolysis of salts, the salts are divided into four parts –
Hydrolysis of salt made from weak acid and strong base
The solution obtained from hydrolysis of salt made from a weak acid and strong base is basic.
Example: Aqueous solution of sodium carbonate is basic. On dissolving Na2CO3 in water, the following actions take place –
H2O ⇌ H+ + OH–
Na2CO3 ⇌ 2Na+ + CO32-
2Na+ + 2OH– ⇌ 2NaOH (strong base)
2H+ + CO32- ⇌ H2CO3
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
NaOH is a strong base and is almost completely ionized. H2CO3 is weak acid and the number of H+ ions obtained from its ionization is less. Hence the number of OH– ions in the solution is high and the solution is basic.
Similarly, CH3COONa and KCN are also salts made from weak acid and strong base and their aqueous solutions are basic.
Hydrolysis of salt made from strong acid and weak base
The solution obtained from hydrolysis of salt made from strong acid and weak base is acidic.
Example: Aqueous solution of ammonium chloride is acidic. The following actions take place when NH4Cl is dissolved in water.
H2O ⇌ H+ + OH–
NH4Cl ⇌ NH4+ + Cl–
NH4+ + OH– ⇌ NH4OH (weak base)
H+ + Cl– ⇌ HCl (strong acid)
HCl is strong acid and almost completely ionized NH4OH is a weak base and the number of OH ions obtained from its ionization is low. It is acidic due to the high number of H ions in the solution.
Similarly Cu and HeSO4 are obtained by dissolving CuSO4 in water. Which are weak base and strong acid respectively. Hence the aqueous solution of CuSO4 is also acidic. Similarly, FeCl3 is also a salt of this category and its aqueous solution is acidic.
Hydrolysis of salt made from strong acid and weak base
CO3 and CH3COONH4 are salts of this category. The solution obtained from hydrolysis of this category of salts can be acidic, basic or neutral. It depends on the relative strength of acid and base.
Example: On dissolving ammonium carbonate in water, the following actions take place.
H2O ⇌ H+ + OH–
(NH4)2CO3 ⇌ 2NH4+ + CO32-
NH4+ + OH– ⇌ NH4OH (weak base)
2H+ + CO32- ⇌ H2CO3 (weak acid)
NH4OH is weak base and H2CO3 is weak acid. The ionization of NH4OH is higher than that of H2CO3. Hence the concentration of OH– in the solution is high and the solution is basic.
On dissolving ammonium carbonate in water, the following actions take place –
H2O ⇌ H+ + OH–
CH3COONH4 ⇌ CH3COO– + NH4+
CH3COO– + H+ ⇌ CH3COOH (weak acid)
NH4+ + OH– ⇌ NH4OH (weak base)
CH3COOH is weak acid and NH4OH is weak base. Their values of Ka and Kb are 1.80 x 10-5 and 1.78 x 10-5 respectively. Hence, their ionisation volumes are almost the same. Hence, the number of H+ and OH– ions in the solution is almost equal and the solution is neutral.
Hydrolysis of salt made from strong acid and strong base
NaCl is the salt in this category. When this salt is dissolved in water, it gets ionized but there is no action with water.
The reason for this is that strong acid and strong base completely dissociate in aqueous solution and no new substance is formed.
Because of this there is no change in the number of H+ and OH– ions and the solution is neutral. Therefore, salt water of this category is not decomposed and their aqueous solution is neutral.