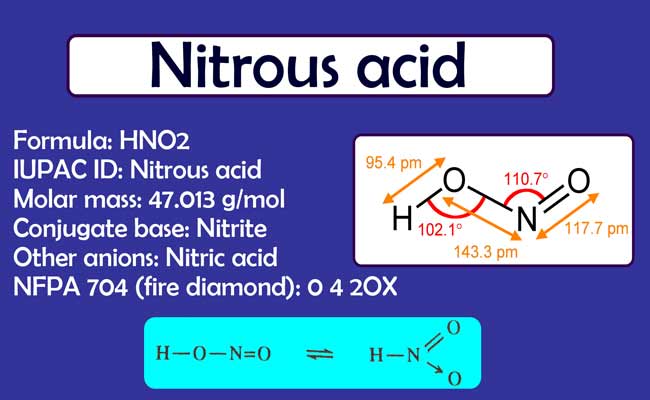
Nitrous acid is a temporary additive. It is not found in an independent state. Its aqueous solution can be obtained at low temperatures. It continues to decompose slowly even at room temperature.
Therefore, to be used in other reactions, first make its solution at low temperature and immediately after that.
Preparation of Nitrous Acid
Nitrous acid is obtained by the action of dilute HCl or H2SO4 on any nitrite.
KNO2 + HCl → KCl + HNO2
NaNO2 + HCl → NaCl + HNO2
KNO2 + H2SO4 → KHSO4 + HNO2
Nitrous acid is obtained when di-nitrogen tri-Oxide is dissolved in cold water.
N2O3 + H2O → 2HNO2
HNO2 is obtained by the action of barium nitrite and cold and dilute H2SO4.
Ba(NO2)2 + H2SO4 → BaSO4 + 2HNO2
This reaction is used in the laboratory method of making nitrous acid.
Barium sulfate is filtered and separated and remains in HNO2 solution.
Physical Properties
The color of its aqueous solution is light blue.
Chemical Properties
Decomposition: Anhydrous nitrous acid is temporary and decomposes into NO, NO2 and H2O.
2HNO2 ⇌ NO + NO2 + H2O
It slowly decomposes in aqueous solution –
3HNO2 → 2NO + H2O + HNO3
In aqueous solution, its decomposition speed is higher at low temperature and high at high temperature.
Acidic Properties: Nitrous acid is a weak acid, it reacts with alkalis to form salts called nitrous salts.
HNO2 + NaOH → NaNO2 + H2O
Oxidizing Properties: In the presence of catabolizes, it decomposes and produces nascent oxygen.
For this reason it is a strong oxidizer.
2HNO2 → 2NO + H2O + O
This iodine is released from any iodide salts in the presence of dilute H2SO4.
2KI + H2SO4 + 2HNO2 → 2NO + I2 + K2SO4 + 2H2O
This oxidizes stannous chloride to stannic chloride.
SnCl2 + 2HNO2 + 2HCl → SnCl4 + 2H2O + 2NO