Ozone: Tests, Uses and Ozone Structure
Ozone Structure
The following experiments have been done to determine the ozone structure. Its structure has been decided on the basis of these experiments.
Sorets Experiment
In this experiment, two graduated flasks of equal volume are filled with oxygen gas containing ozone at the same conditions of temperature and pressure.
After this, these flasks are inverted into a cistern filled with water. By reading the level up to which the water level rises in the flasks, we can find out the volume of the gas filled in the flasks.
Now put turpentine oil in one flask and heat the other flask. The water level in the first flask rises. Due to the decomposition of ozone into oxygen in the second flask, the volume of the gas filled in it increases and due to this the water level in the flask goes down.
The decrease and increase in volume are known by reading the position of the water level in both the flasks. In this experiment it is found that the decrease in volume in the first flask is twice the increase in volume in the second flask.
This proves it. That two volumes of ozone decompose to form three volumes of oxygen. According to Avogedro’s law, equal volumes of gases at the same temperature and pressure have the same number of molecules.
Therefore, 2 molecules of ozone decompose to form 3 molecules of oxygen, that is, 1 molecule of ozone forms 3/2 molecules of oxygen or 3 atoms of oxygen, so the molecular formula of ozone is O3.
Newths Experiment
The equipment used in this experiment consists of two coaxial tubes made of rubber. A thin tube filled with turpentine oil is hung in the middle of one part of these tubes. The outer edge of the second part of these tubes is attached to the manometer, which is filled with colored H2SO4. Newths Experiment
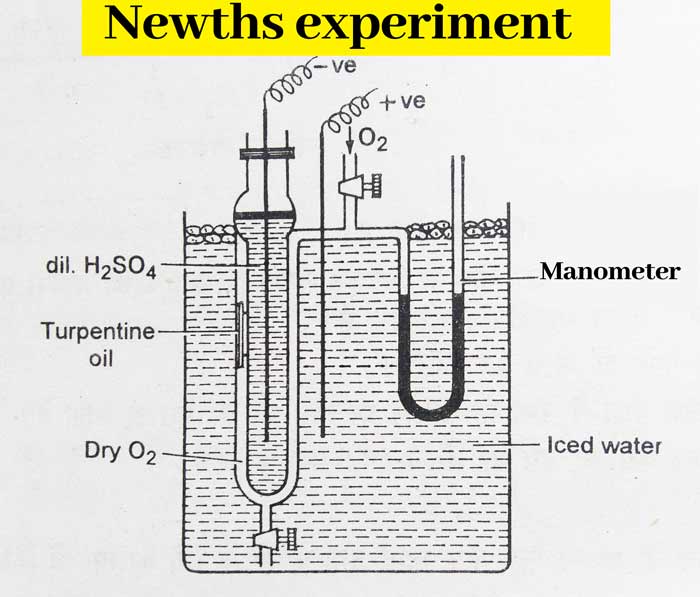
Now the manometer is read by filling O2 in this tube and electric discharge is passed. As a result, oxygen is converted into ozone.
In this process there is a decrease in volume which is detected by manometer. Now the thin tube filled with turpentine oil is broken by rotating the tube outside it.

Turpentine oil absorbs ozone and decreases in volume. It is also detected with the help of manometer.
From this experiment it is concluded that the decrease in the volume of ozone due to the dissolution of turpentine oil is twice that of the reduction in the formation of ozone from oxygen, that is, 3 volumes of oxygen make 2 volumes of ozone, that is, 1 molecule of ozone. Composed of 3 atoms of oxygen. Hence its molecular formula is O3.
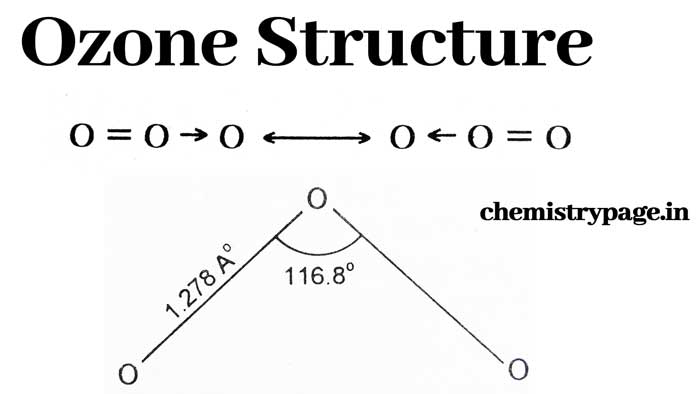
Pauling, shand and sparr suggested in 1943 that ozone is a resonance hybrid of the following two structures.

Based on the discoveries of Trambaruls (1953) and Hughes(1956), its angular structure is proved, according to which its O – O – O angle is 116.8° and the length of O – O bond is 1.278 A°.
Tests of Ozone
Ozone is absorbed by turpentine oil.
When exposed to ozone, mercury tends to stick to the glass. Due to this the meniscus of mercury is destroyed and mercury loses its fluidity.
This starch turns iodide paper blue.
On adding benzidine to an alcohol solution of ozone, the color of the solution turns brown.
With alcoholic tetramethyl base it produces a purple colour.
Uses of Ozone
It is used as a bleach in the bleaching of oils, waxes, starches and fabric dyes.
It is used as a disinfectant.
It is also used to purify water and air.
It is also used in the oxidation and decomposition of many carbonic compounds.
It is also used in the manufacture of silk, camphor and potessium parmagnate.
Difference between Hydrogen Peroxide and Ozone
| Hydrogen Peroxide (H₂O₂) | Ozone (O₃) |
|---|---|
| It is a compound. | It is an allotrope of oxygen. |
| It is colourless, odourless, thick and transparent liquid. Its color appears to be light blue in large quantities. | It is a gas smelling like fish. In the liquid state, its color appears to be dark blue. |
| It is soluble in water. | It is soluble in water in small amounts. |
| Hydrogen peroxide with solution of titanium salts gives orange and yellow colours. Any fluoride blows away this color. | Ozone does not react with a solution of titanium salts. |
| Hydrogen peroxide does not react with benzidine. | On adding Benzidine to an alcoholic solution of ozone, the color of the solution becomes brown. |
| Hydrogen peroxide does not react with tetramethyl base. | On adding tetramethyl base to ozone in an alcoholic solution, the color of the solution turns purple. |
| It is used as an antiseptic. | It is also used as an antiseptic. |
Some more difference between Hydrogen Peroxide and Ozone:
Hydrogen Peroxide
In the presence of sunlight, heat, rough surface etc., it decomposes into water and oxygen.
2H2O2 → 2H2O + O2
A leaf soaked with starch and KI solution turns blue when it comes in contact with it.
2KI + H2O2 → 2KOH + I2
I2 + Starch → Blue colour
Ferric sulfate is formed by the reaction of acidic ferrous sulfate and hydrogen peroxide.
The pink color of KMnO4 disappears due to the reaction of acidic KMnO4 and hydrogen peroxide.
On adding H2O2 to a mixture of acidic potassium dichromate and ether, the color of the ether layer becomes blue due to the formation of blue percromate (CrO5).
Ozone
It decomposes in the presence of silver, palladium, platinum etc.
2O3 → 3O2
A leaf soaked with starch and KI solution turns blue when it comes in contact with it.
2KI + O3 + H2O → 2KOH + I2 + O2
I2 + Starch → Blue colour
Ferric sulfate is also formed by the reaction of acidic ferrous sulfate and ozone.
Acidic KMnO4 and ozone do not react.
There is no reaction of ozone with potassium dichromate.