The existence of eka-aluminum, eka-boron, and eka-silicon, later to be named gallium (Ga), scandium (Sc), and germanium (Ge).
General Properties of “s” block elements
1. Last electron enter in “s” orbitals (exception He and H)
2. Electronic configuration: ns1–2, where n = 2 – 7
3. They are soft metal having low boiling point and melting point
4. They are very reactive, and metallic character increase down the group
5. They form ionic compounds (exception Li and Be)
6. They are highly electropositive and low I.P.
7. Oxidation state +1 and +2
8. They are strong reducing agent
9. They are good conductor of heat and electricity
General Properties of “p” block elements
1. Last electron enter in “p” orbitals (exception He)
2. Electronic configuration: ns2 np1-6
3. They contain metal, semimetal and non metal
4. They form covalent compounds
5. They are highly electronegative and high I.P.
6. They show variable oxidation state
7. They are strong oxidizing agent
8. They are bad conductor of heat and electricity
General Properties of “d” block elements
1. Last electron enter in “d” orbitals
2. Electronic configuration: (n−1)d(1−10) ns(1−2)
3. They contain metal, hard, malleable, ductile, having high boiling point and melting point
4. They show variable oxidation state
5. They form coordination compounds
6. They form coloured compounds
7. They act as catalyst
8. They form alloys
9. They form both covalent and ionic compounds
10. They are good conductor of heat and electricity
General Properties of “f” block elements
1. Last electron enter in “f” orbitals
2. Electronic configuration: (n-2)f(1-14) (n-1)d(0-1) ns2 n = 6-7
3. 4f series called Lanthanoids (58 Ce to 71 Lu)
4. 5f series called Actenoids (90 Th to 103 Lr)
5. After atomic number 92 U, they all are synthetic elements (61-Pm), called transuranic elements
6. They are heavy metal, having high boiling point and melting point
7. They show variable oxidation state
8. They form coordination compounds
9. They form colored compounds
10. all actinides are radioactive
Metals
1. Metals are usually solids at room temperature (Exeption: Hg)
2. Metals usually have high melting and boiling points. [Gallium (Ga) and Caesium (Cs) also have very low melting points (303K and 302K, respectively)].
3. They are good conductors of heat and electricity.
4. They are malleable (can be flattened into thin sheets by hammering) and ductile (can be drawn into wires).
Non-metals
1. Non-metals are located at the top right hand side of the Periodic Table.
2. Non-metals are usually solids or gases at room temperature with low melting and boiling points (boron and carbon are exceptions).
3. They are poor conductors of heat and electricity.
4. Most nonmetallic solids are brittle and are neither malleable nor ductile.
Metalloids
Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb) and Tellurium (Te)
The elements running diagonally across the Periodic Table show properties that are characteristic of both metals and nonmetals. These elements are called Semi-metals or Metalloids.
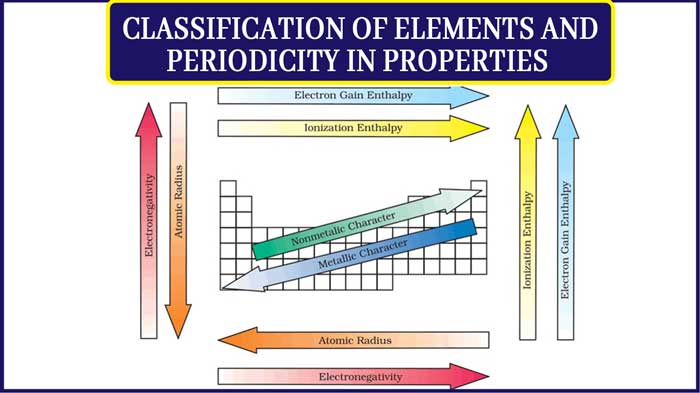
Periodic Properties
1. Atomic radius
2. Ionization enthalpy
3. Electron gain enthalpy
4. Electronegativity
Atomic Radius
Atomic size is the distance between the center of the nucleus of an atom and its outermost shell.
Atomic Radius Variation
General, the atomic radius decreases as we move from left to right in a period and it increases when we go down a group.
Within the period the outer electrons are in the same valence shell
The effective nuclear charge increases as the atomic number increases
The increased attraction of electrons to the nucleus.
Isoelectronic species
Atoms and ions which contain the same number of electrons
Among isoelectronic species, the one with the larger positive nuclear charge will have a smaller radius.
lanthanide contraction
“As the atomic number of lanthanoid increases then their atomic or ionic radii or size decreases gradually is called lanthanoid contraction.”
Causes of lanthanide contraction
1. As the atomic number in lanthanoid series increases
2. Electrons are going to fill in 4f orbital.
3. There is imperfect shielding of electrons in 4f orbital as compared to d orbital
4. Due to imperfect shielding nucleus pull the electrons toward it as a result ionic size or atomic size decreases.