What is alcohol exactly?|| Reactivity order of alcohols
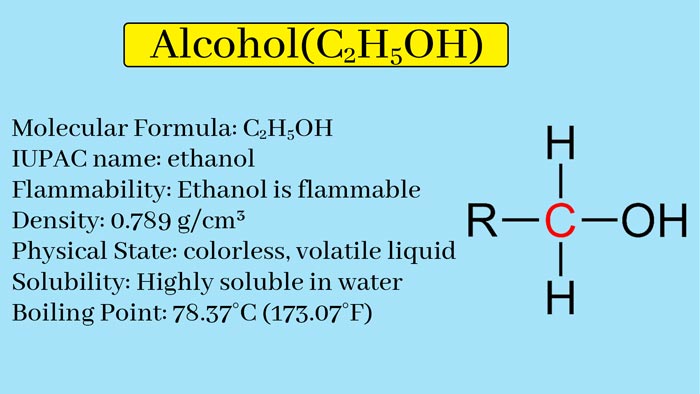
Alcohol Definition
The OH group is called the Hydroxyl or Hydroxyl group. When OH group is attached to an alkyl group, it is called Alcoholic group and the corresponding compound is called Alcohol. CH3OH contains alcoholic group and is called Methyl Alcohol. Similarly, alcoholic group is also present in C2H5OH and it is called Ethyl Alcohol.
Some other compounds in which OH group is present show all reactions of alcoholic group. The OH group present in these compounds is also called Alcoholic group and these compounds are called Alcohol.
The OH group in C6H5CH2OH is also similar to the reactions of OH group present in methyl alcohol and ethyl alcohol. Hence OH group present in C6H5CH2OH is also called alcoholic group. And this compound is called benzyl alcohol.
Similarly, alcoholic group is also present in CH2 = CH – CH2OH and its common name is alkyl alcohol.
Some other compounds in which OH group is present do not exhibit reactions of alcoholic group, these compounds are not called Alcohol.
Example:
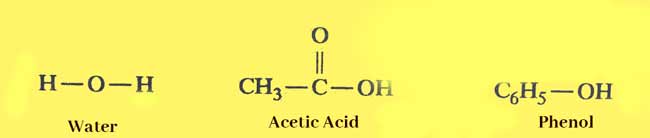
The -OH compounds present in the above compounds but do not contain alcoholic group and are not alcohols.
On inspecting the structures of all the above compounds, it is known that the alcoholic group is always attached to a carbon whose remaining three valencies are satisfied by single binds either by carbon or hydrogen atoms.
Compounds in which the OH group is associated with an alcoholic group are also called mono hydroxy derivatives of alkanes or alkanols in alkenes. Its common formula is ROH. They can be arranged as a homogeneous series. The first member of this category is CH3OH and the second member is C2H5OH.
Classification of Alcohol
Which have only one alcoholic group present, are called Mono hydric alcohols. The alcohols in which two alcoholic groups are present are called Di-hydric alcohols. The alcohols in which three alcoholic groups are present are called tri-hydric alcohols. Alcohols that have more than three alcoholic groups present are called Poly hydric alcohols.
Compounds in which two or more Hydroxy groups are attached to the same carbon atom are usually temporary and one molecule of water is released and makes a permanent additive.

Classification of mono Hydric Alcohols
Alcohols in which the alcoholic group is associated with primary secondary or tertiary carbon atoms are called primary, secondary, and tertiary alcohol respectively. Therefore, in primary alcohols, the alcoholic group is attached to a carbon atom that is not attached to more than one carbon atom.
In secondary alcohols the alcoholic group is attached to a carbon atom that is joined to two other carbon atoms. In tertiary alcohols, the alcoholic group is attached to a carbon atom that is linked to three other carbon atoms.
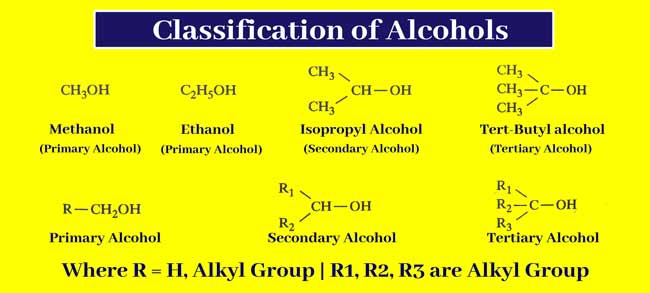
Primary, secondary, and tertiary alcohols are represented by the following general formulas
Where R = H or alkyl group and R1 R2 and R3 are alkyl group.
Nomenclature of Alcohols
There are three methods of naming alcohols. In the ordinary method, they are named on the basis of the name of the group to which the alcoholic group is attached. If the name of this group is methane, then the alcohol related will be named methyl alcohol.
If the name of this group is isopropyl, then the alcohol concerned will be called isopropyl alcohol. Methanol is the name of methanol in the carbinol system. In this method, the names of the other alcohols are written as derivatives of the carbinol.
In the IUPAC method, to name them, the corresponding elkene names add n to the end of the end and the carbon atoms of the carbon atom carrying the OH group. Let’s display the space in the series by the appropriate number.
Isomerism of Alcohols
Alcohol mainly exhibits four types of isomerism
Chain: This type of isomerism is caused by differences in the arrangement of carbon atoms in their chain.
1 butanol
2 Methyl 1 Propanol is a series isomer of each other.
Location: This type of isomerism in alcohols is due to the difference in position of OH group in the chain of carbon atoms.
1 propanol and 2 propanol are interchangeable. Similarly, 1 butanol and 2 butanol are also interchangeable.
Functional isomerism: These display functional isomerism with ethers. Molecular formulas of functional isomers are similar but the functional groups present in them are different.
The molecule formula C4H10O represents a total of 7 structural isotopes. Four of these are alcohol and three ether. Each of these alcohols is the functional isomer of each ether.
Optical isomerism: Alcohols in which the OH group is attached to an asymmetric carbon atom exhibit optical isomerism.
The molecule formula C4H10O represents a total of 7 structural isotopes. Of these, the OH group in the secondary butyl alcohol [CH3CH(OH)CH2CH3] is associated with a carbon atom that is asymmetric. Hence, it also exhibits alcohol optical isomerism as it contains only one asymmetric carbon atom, so its total number of published isomers would be 21 i.e. 2.
Thus the molecule formula C4H10O represents a total of 8 isotopes.
Preparation of Monohydric Alcohol
From Alkyl Halide: Mono hydric alcohols can be obtained by water decomposition of alkhyl hylides by aqueous alkali or liquid oxide silver.
RX + KOH(Liquid) → R-OH + KX
RX + AgOH → R-OH + AgX
C2H5Br + KOH(liquid) → C2H5OH + KBr
CH3Br + AgOH → CH3OH + AgBr
By water Eecomposition of Esters: Alcohol is obtained by conducting water decomposition of esters in the presence of any acid or alkali. The acids used in this reaction are usually sulfuric acid and bases are usually NaOH or KOH.
R-COOR’ + H2O → R-COOH + R’-OH
CH3COOCH3 + H2O → CH3COOH + CH3OH
By water Decomposition of Ether: heating ether with dilute sulfuric acid at high pressure causes their water to decompose and yield alcohol.
R – O – R’ + HOH → R – OH + R’ – OH
CH3 – O – CH3 + HOH → 2CH3OH
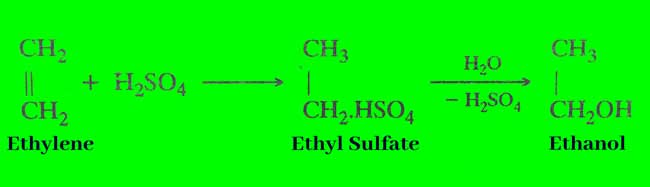
By hydration of alkenes: Alkyle hydrogen sulfate is formed when vapor of alkenes is concentrated in concentrated sulfuric acid. Which are mono-hederic alcohols obtained by steam decomposition? In this whole action, water is added to a molecule of alkenes. Therefore, this action is called hydrolysis of alkenes.
Since elkene is readily available from many sources. Therefore, this method is used in the manufacture of alcohols in commercial quantities.
By the action of Nitrous acid on primary amino: Alcohol is obtained by the action of nitrous acid on primary amines.
R – NH2 + HNO2 → R – OH + N2 + H2O
C2H5NH2 + HNO2 → C2H5OH + N2 + H2O
Nitrous acid is a temporary acid. It is made by the action of sodium nitrite and hydrocloric acid. In order to react with a compound of nitrous acid, this additive is reacted with NaNO2 and HCl.
Nitrous acid reaction on methyl amine gives nitro-methane, methyl nitrite, methyl cyanide and methyl nitrolic acid (NO2.CH=NOH)as a by-product in addition to methanol.
In excess of sodium (Na) and ethanol (C2H5OH) are ketones, Anhydride, carboxylic acid, anhydride, ester, and acid chloride reduced to alcohol.
R – CHO → R – CH2OH
CH3 – CHO → CH3 – CH2OH
R – CO – R’ → R – CH(OH) – R’
CH3 – CO – CH3 → CH3 – CH(OH) – CH3
R – COOR’ → R – CH2OH + R’OH
CH3COOCH3 → CH3CH2OH + CH3OH
Alcohol is also obtained from the reduction of alhydrides, kitones, Carboxylic acid, Ester, Anhydride by H2 / Ni lithium aluminum hydride (LiAlH4) or sodium boro hydride (NaBH4).
CH3CH2COOH → CH3CH2CH2OH
CH3COOCH3 → CH3CH2OH + CH3OH
CH3COCl → CH3CH2OH + HCl
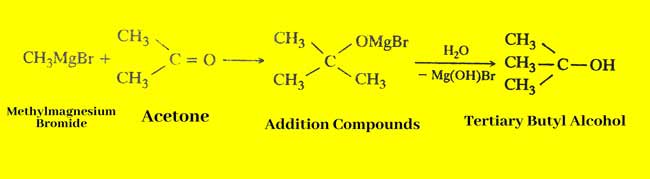
By Grignard Reagent: With the help of Grignard reagent, primary, secondary and tertiary three types of alcohols can be made.
Grignard Reagent
- 1. Reactions with carbonyl compounds
- 2. Reactions with nitrile compounds
- 3. Reactions with esters
- 4. Reactions with cyclic ether
- 5. Reactions with compounds having acidic hydrogen
Primary alcohol: The Grignard reagent reacts with farmeldihides to form additive compounds, whose primary alcohol is obtained by water decomposition.
Primary alcohols can also be obtained by the reaction of Grignard reagent with oxygen or ethylene oxide.

Secondary alcohol: The Grignard reagent also forms additive compounds by reacting with aldyhides other than farmeldihides. They provide secondary alcohol by water decomposition.
Secondary alcohols can also be obtained by the reaction of Grignard reagent with formic acid esters.

Tertiary alcohol: tertiary alcohol is obtained by water decomposition of additive compounds obtained by the reaction of Grignard reagent and kitones.
In addition to Grignard reagent’s formic acid, tertiary alcohol can also be obtained by the reaction with esters of other acids.
Alcohol Properties
Physical properties of Alcohol
Alcohol is a neutral substance.
Physical state: The first three members of the alcohol family are a colorless liquid with a mild odor. They have intense burning taste. Subsequently up to C11H23OH is a thick liquid similar to member oil. High members are odorless solids.
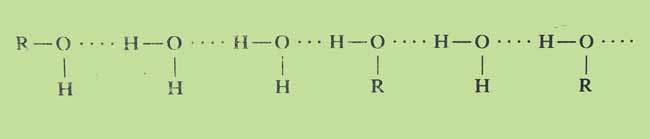
Boiling Point: Alcohol forms molecules associated with hydrogen bonding. Therefore, their boiling point is higher than the boiling point of corresponding alkene.

More energy is required to break associated molecules. Therefore, they have a higher boiling point. Since ethers do not have hydrogen bonding. Therefore, the boiling point of an alcohol is higher than the boiling point of its isomer ether.
As the molecular weight increases, their boiling point and melting point also increase.
The following is the decreasing order of the boiling point in primary secondary and tertiery alcohols.
Primary Alcohol > Secondary Alcohol > Tertiary Alcohol
Solubility: With increasing molecular weight, their solubility decreases in water. The initial members of this class merge more in water because the –OH group present in them forms hydrogen bonds with water molecules. As the chain increases, the characteristic properties of alkenes in alcohols increase as the chain increases. For this reason the solubility of high alcohols in water decreases.
The chain structure of carbon atoms in alcohols also affects their fusion. The straight chain n-butyl alcohol is insoluble in water, but the lateral chain tertiary butyl alcohol is merged in water in every proportion.
Chemical Properties
Mono hydric alcohol is a derivative of alkenes. A mono hydric alcohol is obtained when a hydrogen atom of an alkene is displaced by the –OH group. Mono hydric alcohol can also be considered a derivative of water because a molecule of mono hydric alcohol is obtained by displacing one hydrogen atom from an alkyl radical in a molecule of water.
CH3 – H → CH3 – OH ← H – OH
Example: methyl alcohol is considered to be a hydroxyl derivative of methane and a methyl derivative of water.
The chemical properties of alcohols are mainly based on the -OH group, but they also affect the structure of the alkyl part. There are three types of chemical reactions.
Reactions in which the – OH group is displaced are the functions of the cleavage of the O – H bond.
Reactions in which the entire – OH group is displaced, these reactions occur due to the cleavage of the C – OH bond.
Reactions in which the alkyl group participates. These involve substitution in the alkyl group or elimination of H2O from alcohol.
Reactions in which H of – OH group is displaced:
The speed of these reactions is often as follows –
Primary Alcohol > secondary alcohol > tertiary alcohol
Action from alkali metals: Sodium or potassium metal forms alkoxide by introducing hydrogen atom from -OH group of alcohols.
2ROH + 2Na → 2RONa + H2
2C2H5OH + 2Na → 2C2H5ONa + H2
Carboxylic acid action: Alcohol reacts with carboxylic acid to make ester. This reaction is called esterification. In this reaction, cation of hydrogen (H+) acts as a catalyst and this reaction is reversible. To prevent backward reaction, concentrated anhydrous agents such as H2SO4 or dry HCl are used.
Reaction with Acetyl Chloride or Acetic Anhydride: As a result of the action of alcohols with acetyl chloride, the hydrogen atom of the -OH group of alcohols is displaced by the acetyl group(- COCH3). This reaction is called Acetylation.
CH3COCl + C2H5OH → CH3COOC2H5 + HCl
Alcohols have a similar action with Acetic Anhydride in the presence of sodium acetate.
(CH3CO)2O + C2H5OH → CH3COOC2H5 + CH3COOH
Reaction with Grignard reagents: Reacting with Grignard reagents to make alcohol, alkane.
Reactions in which -OH group is substituted.
The speed of these reactions is usually this way.
Action from ammonia: Alcohol, when heated at high pressure in the presence of zinc chloride, copper chromite or alumina, reacts with ammonia to form primary amine.
C2H5OH + NH3 → C2H5NH2 + H2O
If alcohol is taken in excess, then secondary and tertairy amine are also obtained.
C2H5OH + C2H5NH2 → (C2H5)2NH + H2O
C2H5OH + (C2H5)2NH → (C2H5)3N + H2O
Halogen acids: The alcoholic group is displaced by the halogen atom as a result of the action of alcohols with halogen acids. This reaction with concentrated HCl occurs in the presence of anhydrous zinc chloride.
R – OH + HCl → R – Cl + H2O
C2H5OH + HCl → C2H5Cl + H2O
This reaction with hydro bromic acid is carried out in the presence of concentrated H2SO4.
R – OH + HBr → R – Br + H2O
C2H5OH + HBr → C2H5Br + H2O
This reaction takes place with hydroIodic acid and the alcohol is refluxed with hydroIodic acid to conduct the reaction in advance.
C2H5OH + HI → C2H5I + H2O
They finally form elkene when heated with concentrated hydroIodic acid and red phosphorus.
C2H5I + HI → C2H6 + I2
Phosphorus halides: The alcoholic group is displaced by the helogen atom as a result of the action of the alcohols with phosphorus halides.
C2H5OH + PCl5 → C2H5Cl + POCl3 + HCl
3C2H5OH + PCl3 → 3C2H5Cl + H3PO3
Alcohols have the same action with thionyl chloride(SOCl2). These compounds are formed in the flask itself to react with the phosphorus tri bromide and phosphorus tri iodide of the alcohols. For this, bromine or iodine is added to the mixture of phosphorus and alcohol.
Reaction with sulphuric Acid: Alcohol reacts with concentrated sulfuric acid to produce different products at different temperatures.
Ethyl alcohol and concentrated sulfuric acid react at ordinary temperature and form ethyl hydrogen sulfate. The reaction takes place easily when the temperature increases, that is, the reaction speed increases.
C2H5OH + H2SO4 → C2H5HSO4 + H2O
Distillation of ethyl hydrogen sulfate at low pressure makes it ethyl sulfate.
2C2H5HSO4 → (C2H5)2SO4 + H2SO4
When ethyl hydrogen sulfate is distilled with water, it decomposes to form ethyl alcohol.
C2H5HSO4 + H2O → C2H5OH + H2SO4
A mixture of concentrated sulfuric acid in excess of ethyl alcohol and heating the mixture at 140°C gives diethyl ether. Diethyl ether is also obtained by heating the homogeneous mixture of ethyl alcohol and ethyl hydrogen sulfate at 140°C.
C2H5OH + H2SO4 → C2H5HSO4 + H2O
C2H5OH + C2H5HSO4 → C2H5-O-C2H5 + H2SO4
By heating ethyl alcohol to 160-170°C with concentrated H2SO4 it forms ethylene.
C2H5OH + H2SO4 → C2H5HSO4 + H2O
C2H5HSO4 → C2H4 + H2O
Reactions in which the alkyle group participates.
Dehydration: The dehydration of alcohols can also be done by passing their vapor over heated alumina. By passing ethyl alcohol vapors onto heated alumina, diethyl ether at 250 temperature and ethylene at 360 temperature are obtained.
2C2H5OH → C2H5 – O – C2H5 + H2O
C2H5OH → C2H4 + H2O
Chlorine action: Alcohols are first oxidized as a result of their reaction with chlorine. And aldehyde or ketones are made. This is followed by chlorination of aldehyde or alkyl radical of ketones.
C2H5OH + Cl2 → CH3CHO + 2HCl
CH3CHO + 3Cl2 → CCl3CHO + 3HCl