Redox Reaction class 11 Notes: Download PDF files and DPP
Redox Reaction class 11 Notes
The reaction which involve oxidation
and reduction is known as a redox reaction
| Oxidation | Reduction |
|---|---|
| 1.Addition of oxygen | 1.Addition of hydrogen |
| 2.Removal of hydrogen | 2.Removal of oxygen |
| 3.Addition of electronegative element | 3.Addition of electropositive element |
| 4.Removal of electropositive element | 4.Removal of electronegative element |
| 5.Loss of electron by an element | 5.Gain of electrons by an element |
For more detail join us on Link to Join telegram group https://t.me/joinchat/NC9eVRt24PWWoVp… Link to Join telegram channel https://t.me/sanjaychemistrypage
<iframe width="834" height="469" src="https://www.youtube.com/embed/lFs87ZF2lpM" title="Crash Course NEET/JEE 2021 | Redox Reactions | Full Chapter Revision | Important Concepts of NCERT" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share" referrerpolicy="strict-origin-when-cross-origin" allowfullscreen></iframe>
Oxidation is a process where the oxidation number of an element increases.
Reduction is a process where the oxidation number of an element decreases.
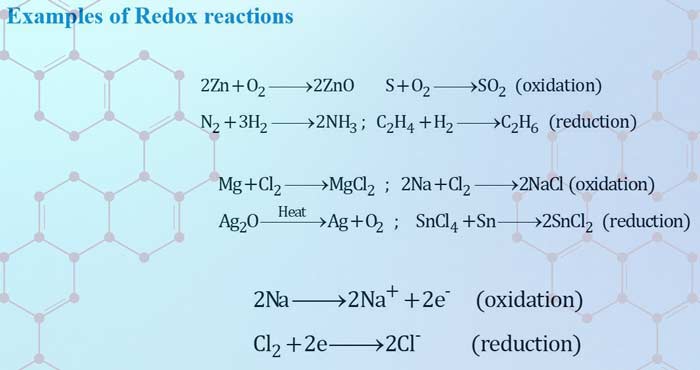
Oxidation is a process where the oxidation number of an element increases.
Reduction is a process where the oxidation number of an element decreases.
Oxidising and Reducing agents
The substance which gets oxidised during a redox reaction is known as reducing agent.
The substance which gets reduced during a redox reaction is known as oxidising agent
Non metal: Oxidising agent
Metal: Reducing agent
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
Identify the substance undergoing oxidation and reduction in the following
1) reactions and Identify the
2) oxidizing and reducing agents
Oxidation number
Oxidation number is the apparent charge possessed by an atom in a molecule
Rules to calculate the oxidation number
1.The oxidation number of an atom in pure elemental form is always zero ( H2, Cl2, Zn, N2, S8, P4 )2.The oxidation number of fluorine is always -1. 3.The oxidation number of alkali metals is always +1 and that of alkaline earth metals is +24.The oxidation number of oxygen in most of the compounds is -2. But in peroxides and superoxides the oxidation state is -1 and -½ respectively5.The oxidation number of hydrogen in most of the compounds is +1. But in metal hydrides like NaH, CaH2 the oxidation number is -1. 6.In a molecule the sum of oxidation numbers of all the elements is zero.7.In a polyatomic ion, the sum of the oxidation numbers of all the atoms is equal to the charge on the ion.
Types of Redox Reactions
There are four types of redox reactions.
- Combination reactions
- Decomposition reactions
- Displacement reactions
- Disproportionation reactions
A combination reaction is a reaction in which elements combine to form the products.
Either of A or B or both A and B must be elemental in nature
A + B ➜ P
Decomposition reaction
1.Decomposition reaction leads to the breakdown of a compound into two or more components at least one of which must be in the elemental state.2.Decomposition reaction is opposite of combination reaction.
Displacement reaction
An ion (or an atom) in a compound is replaced by an ion (or an atom) of another element.
There are two types of displacement reactions.
1.Metal displacement reaction 2.Non-metal displacement reaction
Disproportionation reaction
In a disproportionation reaction, an element in one oxidation state is simultaneously oxidised and reduced.
1.The element in the form of reacting substance is in the intermediate oxidation state2.fluorine does not show a disproportionation tendency
NEET Exams 2024 Chemistry Notes Redox Reactions
Download Redox reaction class 11 Notes
Redox Reactions Practice Set with Solution
Redox reaction class 11 Notes. No registration needed. But don’t forget to follow us our Social Pages
Redox Reactions Online Test for NEET/JEE
