Resonance effect or mesomeric effect || What is resonance effect with example?
Resonance effects in Chemistry
Resonance effect, When some molecules, ions, or free radicals cannot be explained by a single structure (energy value, bond length, chemical properties), we use two or more structural formulas instead of the usual single structural formula this process is called resonance.
The theory of resonance was put forward by the American chemist L. Pauling in the early 1830s. It is a molecular structure theory. He believes that the true structure of a molecule is caused by the resonance of two or more classical valence bond structures.
Resonance theory includes concepts such as delocalized bonds, bond lengths, and bond energies, and represents the resonance of the electronic method of electron delocalization.

Applications of Mesomeric Effect
The advantage of the resonance type is that the electronic type can be used to characterize the distribution position of the charge in the electronic delocalization system. The description is very convenient and practical.
Fundamental Contents
① When more than two Lewis structure formulas can be written according to the valence bond rule for a molecule or ion (the difference is only the distribution of bonds or electrons, but the position of the nucleus is not changed), then the real molecular structure is these Resonance hybrids, that is, the true structure of the molecule is a resonance hybrid.
The resonance hybrid has the characteristics of the above-mentioned structures in total, but no single resonance structure can represent the molecule individually, each resonance structure cannot exist alone.
② Molecules or ions that have resonances are more stable than those without resonances. The more structures that participate in resonance, the more stable the hybrid, especially if the formula with the same structure participates in resonance, the hybrid is the most stable.
③ Among the resonance formulas, the formula with the lowest energy and similar structure occupies the most probability.
④ The concept of resonance energy signifies that the hybrid is more stable than any single resonance structure.
To write the resonance structure formula correctly, the following rules should be met:
1) Only the movement of bonds and electrons is allowed between resonance structural formulas, and the position of the nucleus is not allowed to change.
2) All resonant structural formulas must conform to Lewis’s structural formulas.
3) All resonance structures must have the same number of unpaired electrons. Take Allyl group as an example:
CH2=CH-CH2 → CH2 -CH=CH2.
4) Electron delocalization can often make molecules more stable and have lower internal energy. In order to measure this stability, resonance energy can be used.
The so-called resonance energy is the energy of the actual molecule and the energy of the most stable resonance structure. Poor, taking the benzene molecule as an example.
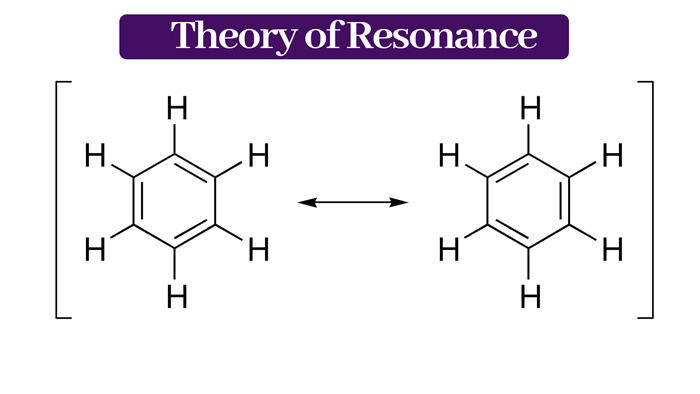
In the resonance structure, the more the number of covalent bonds is, the lower the energy is, the more stable it is. It has a higher probability of the hybrid.
The real structure of benzene is a resonance hybrid composed of eight structural resonances. It should be noted that in the above example, each formula should be a square rectangular carbon ring on a plane. There can be no changes.
These resonance structural formulas are practical the above are hypothetical structures. The difference between them is only in the structure where the electron distribution is not assumed. The difference between them is only in the electron distribution.
Therefore, the energy of each resonance formula is not the same. I and II are similar in structure and have the lowest energy. The remaining resonances have higher energies.
The resonances with the lowest energy and similar structure participate most in the real structure or contribute the most.
Therefore, it can be said that the real structure of benzene is mainly I. Resonance hybrids of formula and formula II.
5) All atoms in the structural formula have a complete valence electron layer and are relatively stable.
6) The stability of charge separation is low.
7) Negative charges are more stable on atoms with greater electronegativity.
Meaning of Resonance Theory
The emergence of Resonance effects first originates from the flaws of the valence bond theory, so what are the flaws of the valence bond theory? Today we all know that benzene has a large Π bond.
In the benzene ring, each carbon atom and all other carbon atoms actually form part of the π bond, except that the π bond with the adjacent carbon atom is a little more, and the distant carbon Atoms have fewer π bonds.
The first point of the valence bond theory is saturation. Since each carbon atom has formed 3 σ bonds, each carbon atom can only form a π bond.
The valence bond theory believes that if each carbon atom has a π bond with an adjacent atom, it can no longer be a π bond with another adjacent atom, let alone a π bond with another atom, and otherwise, it will violate the saturation of the covalent bond.
The reality is that each carbon atom can only form one π bond, but this π bond is formed between all carbon atoms, which means that it is a large Π bond that is delocalized.
In this way, we are very clear about the shortcomings of the valence bond theory: it is a local theory, which stipulates that covalent bonds can only be formed between two atoms.
Therefore, the valence bond theory can accurately and conveniently express the structure of molecules without delocalized large Π bonds (such as ethylene, acetone, etc.) Powerless. In more complicated cases, the valence bond theory is even more difficult to explain, such as O2 It is double radical.
However, the molecular orbital theory developed later did not have
this defect, and the frontier orbital theory proposed by Japanese
chemist Kenichi Fukui in 1951 and the orbital symmetry conservation
principle proposed by American chemists Woodward and Hoffmann in 1965 greatly enriched the molecular orbital theory.
Moreover, in the field of coordination chemistry, the valence bond
theory is completely inferior to the spokesperson of molecular orbital
theory — coordination field theory, so in today’s chemical field,
molecular orbital theory occupies an absolute advantage.
Even so, the valence bond theory is still a favorite of organic chemists (none of them). This is not to say that organic chemists cannot keep up with the times, but because:
(1) the nature of the valence bond theory and the molecular orbital theory are the same, except that the valence bond theory is relatively rough, it fails to be like a molecule The orbital theory considers the arrangement of electrons from the level of the entire molecule but treats the electrons as only the two atoms that form a bond.
(2) The molecular orbital theory is necessarily complicated due to its accuracy. Organic chemists are not structural chemists after all. Solving problems with the complex molecular orbital theory is not their strong point.
The simple valence bond theory has been sufficient for most organic molecules. The need for structural judgment. The valence bond theory is only inaccurate when describing molecules containing large delocalized Π bonds, and it is extremely obvious whether a molecule contains large Π bonds that are delocalized, so it can be used with confidence when there is no large Π bonds.
Pauling deeply understands this hobby of organic chemists, so he pointed out that if a valence bond structural formula must be used to describe a molecular structure containing large Π bonds, then multiple resonance formulas must be combined to represent the structure of a molecule each resonance is a limit structure.
The real structure is an intermediate state between various limits.
For example, the two Kaikoule structures of the benzene ring are two
limit states. Under one limit, there is a complete double bond
between 1, 2 and under another limit, there is a complete single bond between 1, 2. That is true.
Between 1,2 in benzene is between single and double bonds, and that is indeed the case. Therefore, resonance theory clearly expresses a structure that can only be described clearly using molecular orbital theory. It is clearly expressed by the valence bond theory. It is a bridge between molecular orbital theory and valence bond theory. It is a scientific accuracy for scientists. Find a perfect compromise between sex and ease of use.
Mesomeric Effect:
It has a lasting effect. Therefore, this effect is permanently present in the molecules whether they are participating in the reaction or not. It is displayed by M.

Like the electromeric effect, this effect is also found in compounds containing multiple bonds. As a result of this effect, one or more of the pie electron pairs are transferred from one atom to another and exhibit electron transfer with a curved arrow. If one or more solitary electron pairs are present on the atom of the constituent of the additive, the solitary electron pair can also participate in electron transfer.
The reason for electron transfer is that the transfer of electrons results in structures whose internal energy is not much different from the internal energy of the structure. The actual composition of the compounds is the mean of all these structures. This phenomenon is called Mesomerism. And by placing a double-headed arrow between the mesomeric structures, they are displayed.
Example of Mesomeric Effect
Like the electrometric effect, if the displacement of the electrons from one group to the remaining part of the molecule due to the mesomeric effect is called the + M group of that group. If the electron displacement is in its opposite direction, then that group is called -M group. Cl, NH2, OH, and OCH3 groups have a mesomeric effect + M. COOH, CHO, NO2, and SO3H groups have a mesomeric effect – M.
