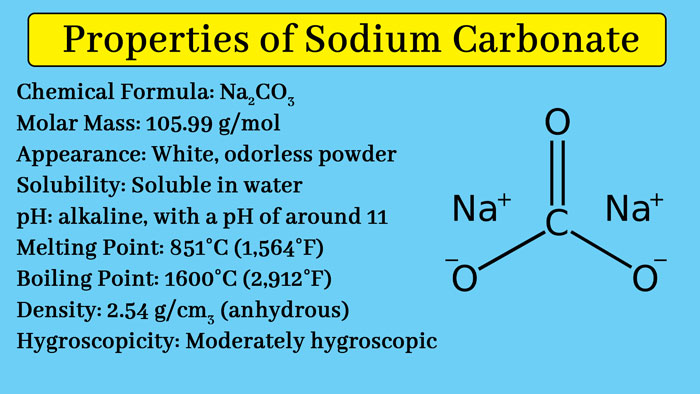
What is sodium carbonate used for? Sodium carbonate properties
- Sodium Chloride – NaCl
- Sodium sulfate – Na2SO4
- Sodium carbonate – Na2CO3
- Calcium Carbonate – CaCO3
In nature, it is found in the form of gentle soil and mortar. The percentage of sodium carbonate in these substances varies from 20 to 50%. There are two main methods of industrial construction.
Leblanc’s Method
Solvay’s ammonia soda method
Leblanc’s Method
In this method, sodium sulfate is made by heating sodium chloride with sulfuric acid.
2NaCl + H2SO4 → Na2SO4 + 2HCl
Sodium carbonate is formed when sodium sulfate is heated with carbon and Calcium Carbonate(CaCO3).
This method divided into deferent steps
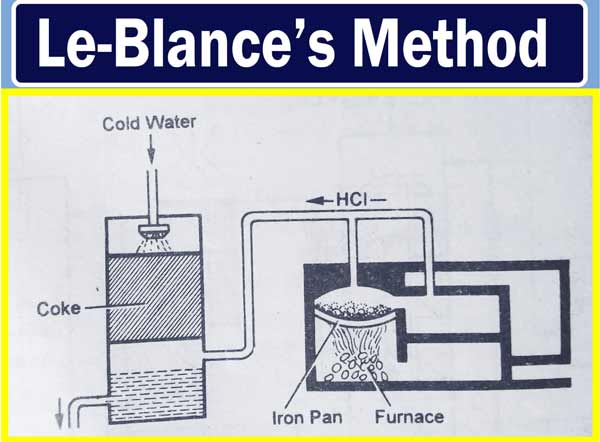
Salt Cake:
This mixture is heated by taking an aqueous solution of ordinary salt in iron Pan and the appropriate amount of concentrated sulfuric acid. Sodium sulfate is removed by the action of sodium chloride and sulfuric acid. And the vapor of HCl is obtained. Thus the HCl obtained, merges into the cold water to form of HCl acids. On more heating the mixture, the remaining amount of sulfuric acid decomposes and separates into a vapor. And sodium sulfate is obtained as a hard solid. This hard solid is called salt cake.
2 NaCl + H2SO4 → Na2SO4 + 2 HCl
Black ash:
The salt cake is broken into small pieces and heated in a revolving furnace with limestone(CaCO3) and cok(C). And becomes sodium carbonate. The mixture obtained from this furnace is called black ash.
Na2SO4 + 4C → Na2S + 4CO
Na2S + CaCO3 → CaS + Na2CO3
Final Reaction
Na2SO4 + 4C + CaCO3 → CaS + Na2CO3 + 4CO
Separating sodium carbonate from black ash:
Black ash mainly contains sodium carbonate. But there are also impurities of Coc, limestone, calcium sulfide, etc. To get sodium carbonate from it, it is very lixiviated by putting it in water. In doing so, sodium carbonate dissolves in water, and impurities are bathed down. Which are filtered and separated. By concentrating the filtered solution, crystals of sodium carbonate are obtained.
Physical Properties
Sodium carbonate is a white colored solid. Its melting point is 8500C. It is a fusion of water. Upon concentration of its aqueous solution, crystals of sodium carbonate are obtained. When kept open in the air, most of its crystal water enters the atmosphere and forms Na2CO3.H2O. Na2CO3 is also called soda ash and Na2CO3.H2O is also called Washing soda.
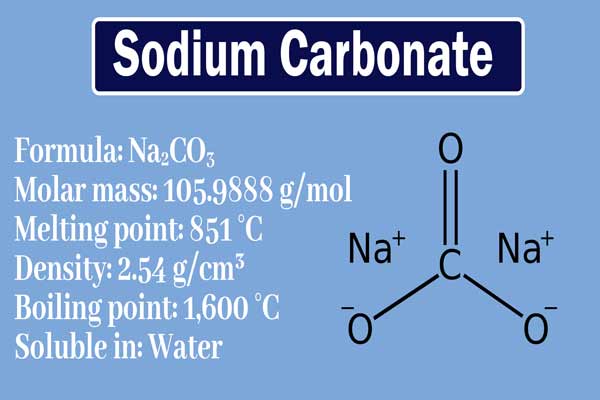
Chemical Properties:
It is an alkaline salt. It dissolves in aqueous solutions to form NaOH and organic acids. NaOH is a strong alkali, the aqueous solution of sodium carbonate is alkaline.
Na2CO3 ⇌ 2Na+ + CO3—
2H2O ⇌ 2H+ + 2OH–
2Na+ + 2OH- ⇌ 2NaOH
2H+ + CO3— ⇌ H2CO3
Acting with acids: Being alkaline, it reacts with acids to form their corresponding salts. This reaction is completed in two terms.
Na2CO3 + HCl → NaHCO3 + NaCl
NaHCO3 + HCl → NaCl + H2O + CO2
Action with acidic oxides: Like acids, it also reacts with acidic oxides to form salts.
Na2CO3 + H2O + CO2 → 2NaHCO3
Na2CO3 + 2SO2 + H2O → 2NaHCO3 + CO2
Effect of heating: Crystallization water is separated by heating the crystalline of sodium carbonate. It melts when heated. But does not decompose.
Precipitation of metallic carbonates: Calcium carbonate precipitates by heating sodium carbonate solution with slaked lime. And sodium hydroxide is obtained. Carbonate salts of those metals are obtained by adding sodium carbonate to aqueous solutions of the salts of most metals. Except for carbonate of alkali metals and ammonium carbonate, all other carbonates are immovable in water and are obtained as precipitates.
Na2CO3 + Ca(OH) → 2NaOH + CaCO3
Na2CO3 + CaCl2 → 2NaCl + CaCO3
Na2CO3 + Ba(NO3)2 → 2NaNO3 + BaCO3
Na2CO3 + BaCl2 → 2NaCl + BaCO3
Salts of some metals precipitate their hydroxide upon adding sodium carbonate to the aqueous solution.
2FeCl3 + 3Na2CO3 + 3H2O → 2Fe(OH)3 + 6NaCl + 3CO2
2AlCl3 + 3Na2CO3 + 3H2O → 2Al(OH)3 + 6NaCl + 3CO2
2CrCl3 + 3Na2CO3 + 3H2O → 2Cr(OH)3 + 6NaCl + 3CO2
Some metals salts have their organic precipitates when it is added to an aqueous solution.
3ZnSO4 + 3Na2CO3 + H2O → 2ZnCO3.Zn(OH)2+ 3Na2SO4 + CO2
3Pb(NO3)2 + 3Na2CO3 + H2O → 2PbCO3.Pb(OH)2+ 6Na2NO3 + CO2
The aqueous solutions of these salts are heated with sodium bicarbonate to form carbonates of these metals.
ZnSO4 + 2NaHCO3 → ZnCO3+ Na2SO4 + CO2 + H2O
ZnCl2 + 2NaHCO3 → ZnCO3+ 2NaCl + CO2 + H2O
PbSO4 + 2NaHCO3 → PbCO3+ Na2SO4 + CO2 + H2O
Uses of Sodium Carbonate:
- As a reagent in the laboratory
- In washing clothes and making soap
- In removing the hardness of water
- Making soda
- In the Glass and paper industry
- In metallurgy of many metals
- Making dyes