Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
Sodium chloride is an ionic compound with the chemical formula NaCl. It is a colorless cubic crystal or fine crystalline powder and tastes salty.
The appearance is a white crystal and its source is mainly sea water, which is the main component of table salt. Soluble in water, glycerin, slightly soluble in ethanol (alcohol), liquid ammonia, insoluble in concentrated hydrochloric acid.
Impure sodium chloride is deliquescent in air.
The stability is relatively good. Its aqueous solution is neutral.
Industrially, the method of electrolytically saturated sodium chloride solution is generally used to produce hydrogen, chlorine and caustic soda (sodium hydroxide) and other chemical products (commonly called Chlor-alkali industry).
Important Properties of Common Salt
Basic Information
English name: Sodium Chloride
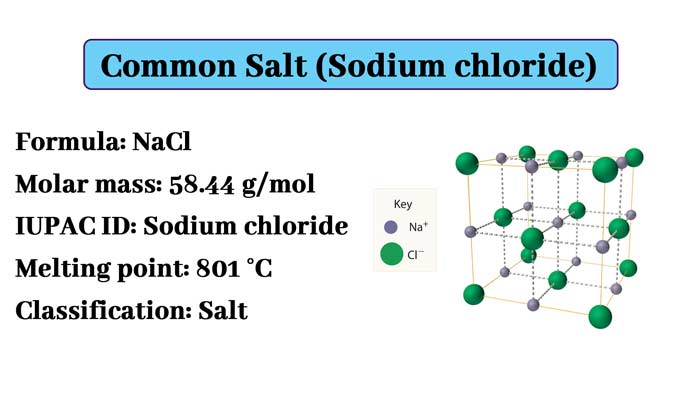
Molecular formula: NaCl
Molecular weight: 58.44280
Physical Properties :-
It is a colorless crystal solid material.
It is highly soluble in water.
Its taste is salty.
It cracks when heated.
When it left open in the air, it exhales after getting moisture. This happens due to the impurity of magnesium chloride in the salt.
Its solution is neutral towards litmus.
The density of pure sodium chloride is 2.17 and the melting point is 800°C.
NaCl is dispersed in alcohol to form colloids. Its solubility in water is reduced by the presence of hydrogen chloride and it is almost insoluble in concentrated hydrochloric acid.
Chemical Properties of Sodium Chloride
The crystals of sodium chloride form stereo symmetry. In its crystal structure, the larger chloride ions form the densest packing in the cube and the smaller sodium ions fill the octahedral gap between the chloride ions.
Each ion is surrounded by six other ions. This structure is also found in many other compounds, called sodium chloride type structure or stone salt structure.
Reaction with acids :- On heating sodium chloride with concentrated sulfuric acid (H2SO4), hydrochloric acid (HCl) is formed.
NaCl + H2SO4 → NaHSO4 + HCl
NaHSO4 + NaCl → Na2SO4 + HCl
Reaction with silica (sand) :– On heating sodium chloride with silica (SiO2) at high temperature, sodium silicate is formed.
2NaCl + SiO2 + H2O → Na2SiO3 + 2HCl
Reaction with ammonia :- By passing ammonia and carbon hydroxide gas in a concentrated solution of salt, sodium bicarbonate (NaHCO3) and ammonia chloride (NH4Cl) are formed.
NaCl + NH3 + CO2 + H2O → NaHCO3 + NH4Cl
Reaction with manganese dioxide : – Chlorine gas (Cl2) is obtained when it is heated with manganese dioxide and sulfuric acid. Industrial production of chlorine is done by this process.
2NaCl + MnO2 + 3H2SO4 → MnSO4 + 2NaHSO4 + 2H2O + Cl2
Reaction with silver nitrate :- On reacting with silver nitrate solution gives white precipitate of silver chloride.
NaCl + AgNO3 → NaNO3 + AgCl
Preparation of Sodium Chloride
Industrial method
Introduced into salt fields by seawater (average of 2.4% sodium chloride) and dried in the sun, Concentrated to crystallize to obtain a crude product. The seawater can also be heated by steam, filtered by a sand filter and concentrated by ion-exchange membrane electrodialysis to obtain brine (containing sodium chloride 160-180g/L).
The halo gypsum is precipitated by evaporation, and the obtained chlorine is centrifuged. More than 95% sodium (2% moisture) can be dried to obtain common salt. Rock salt and salt lake brine can also be used as raw materials and dried in the sun to obtain raw salt.
When underground brine and well salt are used as raw materials, they are concentrated by three- or four-effect evaporation, crystals are precipitated, and centrifuged to obtain.

Laboratory method
Raw salt is dissolved in water to remove insoluble impurities. Refined preparations such as sodium hydroxide and sodium carbonate are mixed to form soluble impurities such as SO42-, Ca2+ and Mg2+. The precipitate is formed and is filtered to remove it. Finally purified with pH. The solution was concentrated to obtain pure sodium chloride crystals and adjusted to 7 with hydrochloric acid.
The preparation method in the laboratory is to mix an equal amount of hydrochloric acid with sodium hydroxide to form a sodium chloride solution. The solution was then distilled to obtain sodium chloride crystals. The main reaction:
NaCl + AgNO3 → NaNO3 + AgCl
In addition, sodium chloride will also produce sodium chloride when ignited in the environment of chlorine gas. Its chemical equation is:
2Na + Cl2 → 2NaCl
Uses of Salt (Sodium Chloride)
In cooking
In policing the pottery.
To preserve meat, fish, butter, pickles etc.
In making various chemical substances like – washing soda, coasting soda, sodium sulfate, hydrochloric acid, chlorine etc.
in soap making
To prepare a freezing mixture by mixing sodium chloride with ice for making kulfi etc.
