Solutions Short Notes and Previous Year papers with solutions
Solutions short notes. solution chapter in chemistry class 12 notes pdf download the PDF file below Link:
Solutions:
A perfectly homogeneous mixture of two or more components is called a solution.
Solute: The component which is present in a lesser amount or whose physical state is changed during the formation of the solution is called the solute.
Solvent: The component which is present in a larger amount and determines the physical state of the solution is called the solvent.
- Lithium Element || How Lithium Ion Batteries Work || Use of Lithium
- What Helium is Used for || Helium Isotopes || How is Helium Made
- Uranium Uses || What is Uranium Used for || How is Uranium Formed
- Platinum alloys || What Platinum is Used for || Platinum Chemical Properties
- Ether Uses || Why Ether is Insoluble in Water || How Ether is Formed
- Magnesium Benefits || Why Magnesium is so Good for You and Properties
Types of the solution: Depending upon the nature of solute and solvent, solutions are classified as follows:
Gaseous solutions: Solutions in which gas acts as a solvent are known as gaseous solutions.
Liquid solutions: Solutions in which liquids are present in larger amounts.
Solid solutions: Solutions in which solids are present in larger amounts.
Solubility: Maximum amount of substance that can be dissolved in a specified amount of solvent at a specified temperature is called its solubility.
Factors affecting solubility of a solid in a liquid:
Nature of solute and solvent: Polar solutes dissolve in polar solvents and non-polar solutes in non-polar solvents. (i.e., like dissolves like).
Effect of temperature:
– If the dissolution process is endothermic (DsolH > 0), the solubility increases with rise in temperature.
– If dissolution process is exothermic (DsolH < 0), the solubility decreases with rise in temperature.
Effect of pressure: Pressure does not have any significant effect on solubility of solids in liquids as these are highly incompressible.
Factors affecting solubility of a gas in a liquid:
Effect of pressure: Henry’s law states that “the partial pressure of the gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution” p = KH x where, KH is the Henry’s law constant and is different for different gases at a particular temperature.
Higher the value of KH at a given pressure, the lower is the solubility of the gas in the liquid.
Effect of temperature: As dissolution is an exothermic process, then according to Le Chatelier’s principle, the solubility should decrease with increase of temperature.
Raoult’s law: It states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. p1 = p°1 x1 and p2 = p°2 x2 where p°1 and p°2 are vapour pressures of pure components 1 and 2 respectively, at the same temperature.
Dalton’s law of partial pressures:
Ptotal = p1 + p2 = x1 p1° + x2 p2°
= (1 – x2)p1° + x2 p2°
= p1° + (p2° – p1°)x2
If y1 and y2 are the mole fractions of the components 1 and 2 respectively in the vapour phase then, p1 = y1 Ptotal and p2 = y2 Ptotal
Raoult’s law for solid-liquid solutions: It states that relative lowering in vapour pressure of a solution containing a non-volatile solute is equal to the mole fraction of the solute.
p°− ps / p° = x2 where,
p° = vapour pressure of pure solvent
ps = vapour pressure of solution
x2 = mole fraction of solute.
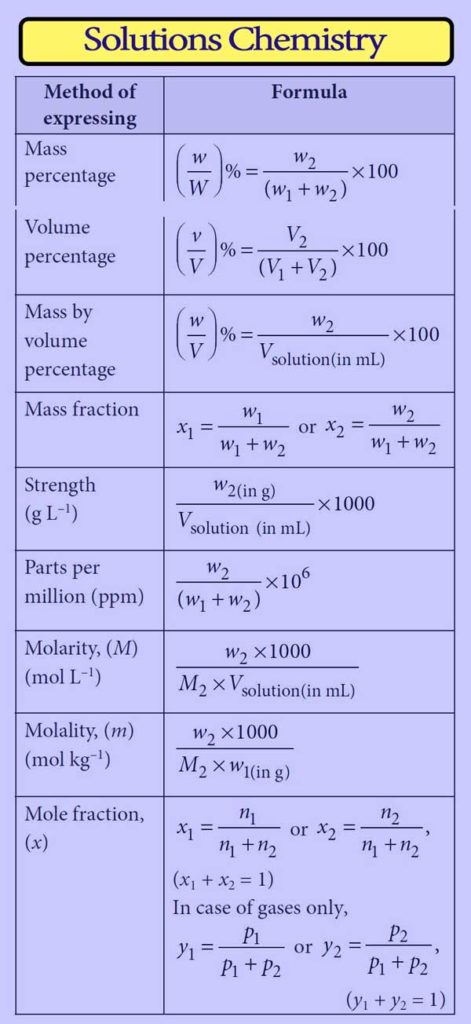
Certainly! Here are some key formulas and concepts in solutions chemistry:
Molarity (M):
Molality (m):
Mass Percentage (% w/w):
Volume Percentage (% v/v):
Parts per Million (ppm):
Dilution Equation:
Colligative Properties: These depend only on the number of solute particles, not their identity. Examples include:
- Boiling Point Elevation:
- Freezing Point Depression:
- Osmotic Pressure:
Raoult's Law:
Henry's Law:
Solvation: Process of solvent molecules surrounding and interacting with solute particles.
