Substitution reaction mechanism || Electrophilic Substitution Reaction In Benzene
Substitution reaction mechanism
Substitution
reactions are a type of chemical reaction where one atom or group of atoms is
replaced by another atom or group of atoms within a molecule. There are two
main types of substitution reactions: nucleophilic substitution and
electrophilic substitution. Here is a brief overview of both mechanisms:
1. Nucleophilic Substitution Reaction Mechanism:
- Definition: In nucleophilic substitution reactions, a
nucleophile (an electron-rich species) attacks and replaces a leaving group (an
atom or group that departs with its pair of electrons) in a molecule.
- Steps:
1. Nucleophile Attack: The nucleophile
attacks the substrate molecule, targeting the atom or group to be replaced.
2. Leaving Group Departure: The leaving group departs, taking its pair of
electrons.
- Common
Types:
- SN1
(Substitution Nucleophilic Unimolecular): In SN1 reactions, the leaving group departs
first, forming a carbocation intermediate. The nucleophile then attacks the
carbocation.
- SN2 (Substitution Nucleophilic Bimolecular): In SN2 reactions, the nucleophile attacks the substrate at the same time the leaving group departs. This results in a concerted reaction with a single transition state.
2. Electrophilic Substitution Reaction Mechanism:
- Definition:
In electrophilic substitution reactions,
an electrophile (an electron-seeking species) replaces a hydrogen atom or
another atom in a molecule.
- Steps:
1. Generation of Electrophile: An electrophile is generated, often through
the loss of a leaving group or activation of a reactant.
2. Attack of Electrophile: The electrophile attacks the substrate
molecule, replacing an atom or group.
- Common
Types:
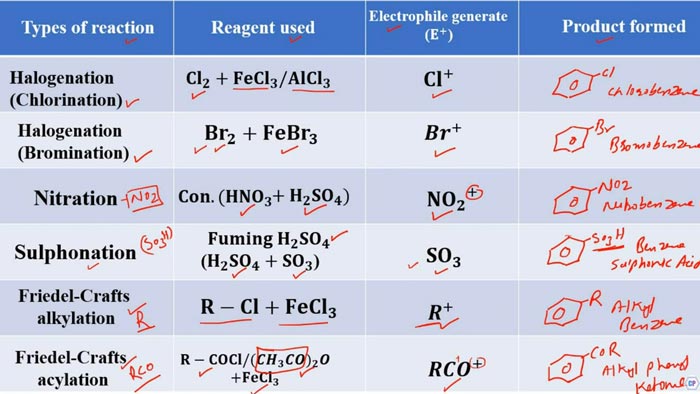
- Aromatic Electrophilic Substitution (SEAr):
In SEAr reactions, an electrophile
substitutes for a hydrogen atom on an aromatic ring. Common examples include
Friedel-Crafts alkylation and Friedel-Crafts acylation.
- Aliphatic Electrophilic Substitution: In aliphatic electrophilic substitution, an
electrophile replaces a hydrogen or another atom in an aliphatic (non-aromatic)
compound.
Example: SN2 Nucleophilic Substitution
Reaction
1. Reactants:
- Substrate: (CH3Cl) (methyl chloride)
- Nucleophile: (OH-) (hydroxide ion)
2. Mechanism:
- The nucleophile -(OH- attacks the
carbon atom in (CH3Cl).
- Simultaneously, the leaving group (Cl)
departs.
- The result is the formation of (CH3OH) (methyl alcohol).
CH3Cl + OH- → CH3Cl + Cl-
Understanding substitution reaction mechanisms is crucial for predicting reaction outcomes, designing synthetic routes in organic chemistry, and explaining the behavior of different functional groups. The choice of reaction conditions, the nature of the reactants, and the surrounding environment all play a role in determining the specific mechanism that will be followed in a substitution reaction.
Nucleophilic Substitution (SN2):
- Reaction:
- Mechanism:
- The hydroxide ion () attacks the carbon atom in methyl chloride ().
- Simultaneously, the leaving group (chlorine) departs.
- The result is the formation of methyl alcohol ().
Nucleophilic Substitution (SN1):
- Reaction:
- Mechanism:
- The bromine atom departs, forming a tertiary carbocation ().
- The hydroxide ion () then attacks the carbocation.
- The result is the formation of tert-butyl alcohol ().
Aromatic Electrophilic Substitution (SEAr):
- Reaction:
- Mechanism:
- The bromine molecule is activated, generating an electrophile ().
- The electrophile attacks the benzene ring, replacing one of the hydrogen atoms.
- The result is the formation of bromobenzene ().
Friedel-Crafts Alkylation (Aliphatic Electrophilic Substitution):
- Reaction:
- Mechanism:
- Aluminum chloride () activates , generating a methyl cation ().
- The methyl cation attacks the benzene ring, replacing one of the hydrogen atoms.
- The result is the formation of toluene ().
Friedel-Crafts Acylation (Aromatic Electrophilic Substitution):
- Reaction:
- Mechanism:
- Aluminum chloride () activates , generating an acylium ion ().
- The acylium ion attacks the benzene ring, replacing one of the hydrogen atoms.
- The result is the formation of acetophenone ().
These examples illustrate different types of substitution reactions, including nucleophilic substitution (SN1 and SN2) and electrophilic substitution (SEAr and Friedel-Crafts). The specific reaction conditions and reagents determine the type of substitution mechanism that occurs.