What is toluene used for? Preparation and Properties
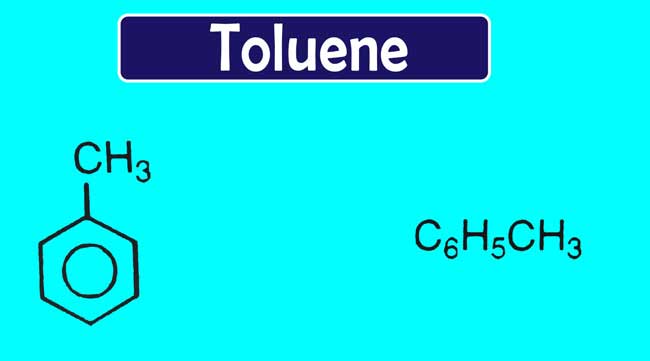
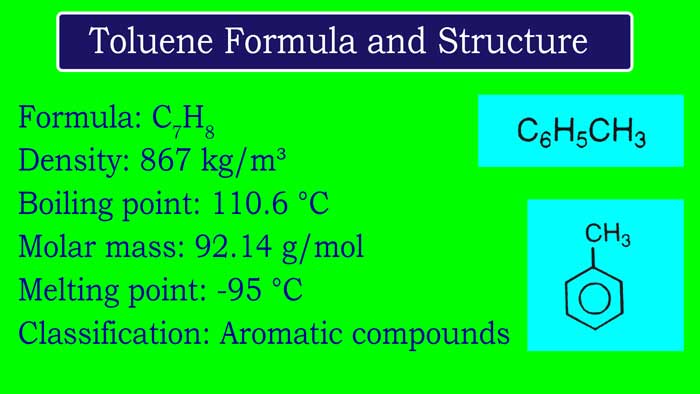
Toluene Formula and Structure
Toluene is an aromatic hydrocarbon. Toluene is a colourless liquid and water-insoluble liquid. It is smell like paint thinners. Toluene IUPAC systematic name is Methylbenzene. Toluene was first derived from reagent named Tolu Balsam. Hence it was named toluene. Toluene formula is C6H5CH3.
Preparation
of Toluene
Benzene
Benzene: toluene can be made from
benzene by a Friedel–Crafts reaction.
C6H6 +
CH3Cl → C6H5CH3 +
HCl
Bromobenzene
Bromobenzene: Toluene is obtained when
sodium is reacted with an analogous mixture of bromobenzene and bromomethane in
ethereal solution.
C6H5Br
+ 2Na + BrCH3 → C6H5CH3 +
2NaBr
Similarly, toluene is also obtained by the action of
chlorobenzene and methyl chloride from sodium. This reaction is called Wurtz Fittig Reaction.
Read More about Wurtz Fittig Reaction – Click Here
Sodium Toluate
Sodium
Toluate: Toluene
can be obtained by heating a mixture of sodium
toluate and soda lime.
C6H4(CH3)COONa
+ NaOH → C6H5CH3 +
Na2CO3
Grignard Reagent
Grignard
Reagent: toluene
is obtained by reaction of phenylmagnesium chloride, bomomide or iodide with
methyl chloride, bromide or iodide.
C6H5MgBr
+ CH3Br → C6H5CH3 +
MgBr2
Coal tar
It is obtained from the efficient distillation of Coal tar in
commercial quantities.
When distillation of Coal tar at 170°C a fractional liquid
obtained. It is called light oil. It is lighter than water.
In addition to benzene, toluene and xylene, basic substances
like pyridine and acidic substances like phenol and cresol are present. To
remove them, light oil is first reacted with sodium hydroxide, which removes
acidic impurities.
After this, its diluents are reacted with H2SO4,
which results in the separation of basic substances such as pyridine.
Now the light oil is washed with water. Due to which acidic
elements are removed. In this way, efficient distillation of purified oil is
done, which gives the following effect.
90% benzoyl (80 – 110°C) – 90% benzoyl means that
distillation of 100 ml at 100°C gives 90 ml distillate. It contains 70% benzene
and the rest toluene and xylene.
50% benzoyl (110 – 140°C): 50% benzoyl means that
distillation of 100 ml at 100°C gives 50 ml distillate, it contains 45% benzene
and the remaining toluene, xylene and other hydrocarbons.
Solvent naphtha or benzoic 140
– 170°C – It is
used in dissolving dyes, rubber and resins, on re-effecting distillation of the
above constituents, benzene at 80°C gives toluene at 110°C and xylene at 135°C.
Petroleum
Petroleum: In commercial quantities it is obtained from
aromatization of n-heptane obtained from petroleum.
It’s C6 – C8 component is heated at high pressure and high temperature in the presence of appropriate catalysts to form benzene, toluene and xylene from petroleum. In this action, benzene, toluene and xylene are obtained as a result of aromatisation of aliphatic and alicyclic hydrocarbons. This action is called catalytic reforming or plat forming. Its ingredients are separated by Fractional distillation from a mixture of benzene, toluene and xylene.

Toluene Physical Properties
·
Toluene is a colourless liquid.
·
Its boiling point is 110.
·
Its smell is similar to benzene
·
It is insoluble in water but soluble in
alcohol, ether and benzene.
·
It is itself a good solvent for many organic
compounds.
·
Toluene Formula: C7H8
Toluene Chemical Properties
Replacement of hydrogen
atoms of benzene ring: In the absence
of sunlight and in the presence of a halogen carrier such as Fe and FeCl3, it reacts with the halogens to form the replacement product.
The methyl group is ortho and para is the
director group. Therefore, in this reaction, hydrogen atoms of ortho and para
positions of methyl group are displaced by halogen atoms.
Example: A mixture of ortho and para substitution products is obtained from mono chlorination of toluene.

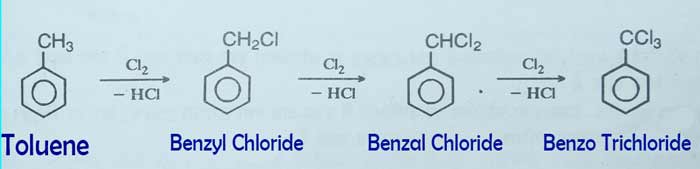
Replacement of hydrogen atoms of methyl group: On flowing
chlorine in boiling toluene in the presence of sunlight, hydrogen atoms
of methyl group are displaced by chlorine atoms and the side chain
substitution product is obtained.
Nitration
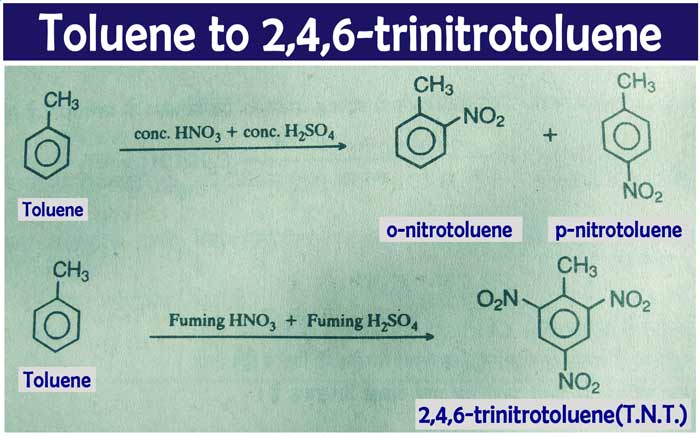
Nitration: A mixture of e-nitro-toluene and p-nitro-toluene is
obtained from the reaction of concentrated HNO3 with toluene in the
presence of concentrated H2SO4.
2,4,6 tri-nitro-toluene(T.N.T.) is obtained by heating toluene at about 50°C
with mild nitric acid and mild sulphuric acid. Which is a strong explosive
substance.
Sulphonation
Sulphonation: A mixture of n- and p-toluenesulfonic acid is obtained by heating toluene with concentrated sulfuric acid or by replicating with dilute sulfuric acid.
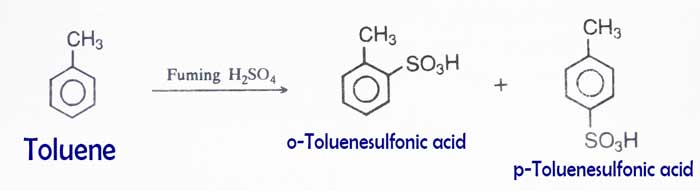
Friedel–Crafts reaction
Friedel–Crafts reaction: A mixture of ortho and para xylene is obtained when the toluene is replicated with methyl chloride in the presence of anhydrous AlCl3.
This reaction is similar to CH3COCl in the presence of anhydrous AlCl3 of toluene and a mixture of ortho and para substitution products is obtained.

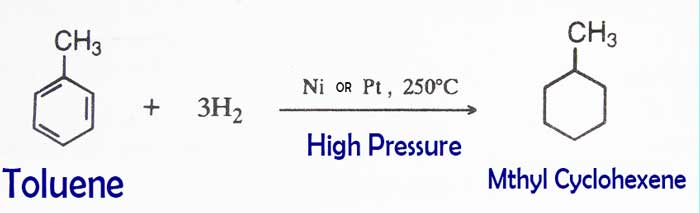
Addition of Hydrogen: In the presence of nickel or
platinum, methyl cyclohexane is obtained as a result of high pressure
and the addition of toluene and hydrogen at about 250°C temperature.
Oxidation: (i) It is benzaldehyde oxidized by chromyl chloride. This reaction takes place in two steps.
C6H5OH3 + 2CrO2Cl2 → C6H5CH(OCrOHCl2)2 → C6H5CHO
This reaction is called etard’s reaction. Oxidation of toluene from CrO3 and acetic anhydride also gives benzaldehyde.
benzaldehyde is also obtained by oxidation of toluene by an acidic solution of magazine dioxide at 350°C. Oxygen of toluene by oxidation of air in the presence of vanadium pentoxide (V2O5) also gives benzaldehyde.
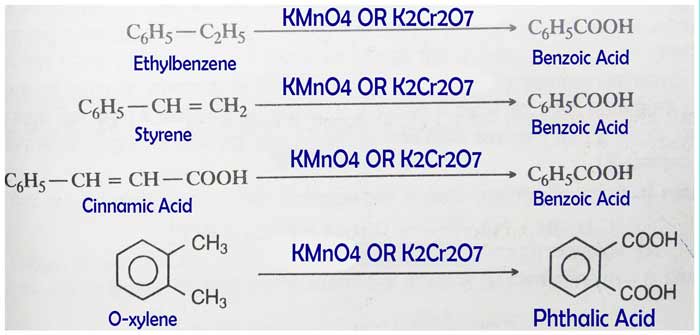
It is benzoic acid oxidized by acidic KMnO4 or acidic K2Cr2O7.
C6H5OH + 3O → C6H5COOH + H2O
Important Notes: If a lateral chain (-CH3, -C2H5, -CH=CH2) is present in an aromatic compound, the entire lateral chain is converted into the -COOH group upon oxidation of that compound by acidic KMnO4 or acidic K2Cr2O7.
The oxidation of tertiary butyl benzene is a consequence of the above general trend of aromatic compounds. The main product of oxidation by its acidic KMnO4 or acidic K2Cr2O7 is not benzoic acid. In this reaction, the product formed by fission of benzene ring is the main product.
Toluene Uses
It is used in making many other aromatic compound, which are used as pigments, pigments, explosives and other useful substances. Of these, T N T and securin are the two main and useful substances obtained from toluene.
It is used as oil fat rubber and some other substances.
It is also used in dry cleaning of clothes.
Toluene can be used as fuel in moter cars in combination with petrol.