What is Acetaldehyde made of? | Properties, uses, and Tests
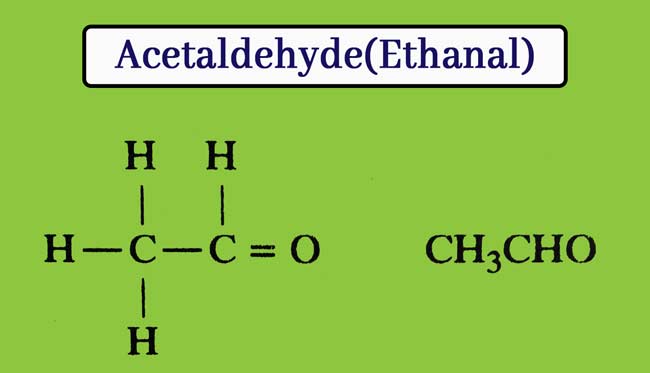
This compound is the second member of aldehyde. Its molecule formula C2H4O and structure formula are as follows.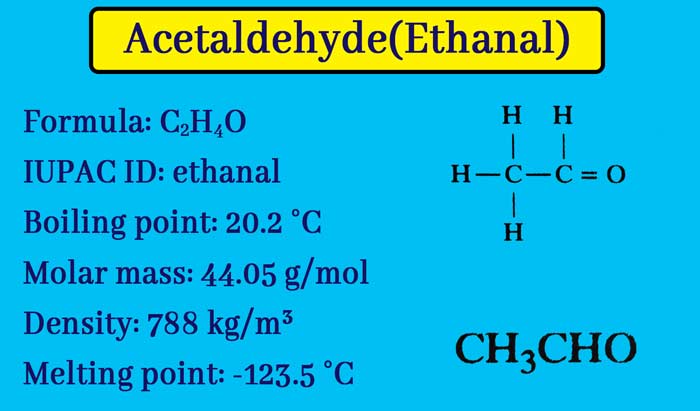 Preparation
PreparationIn the laboratory acetaldehyde is made by oxidizing ethyl alcohol with acidic potassium dichromate.
K2Cr2O7 + 4H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3O
CH3CH2OH + O → CH3CHO + H2O
The acetaldehyde derived from this reaction can be readily oxidized to acidic potassium dichromate to form acetic acid, but the conditions of the reaction are arranged such that the product is acetaldehyde and cannot be oxidized.
For this, the reaction is heated by mixing and the acetaldehyde obtained from the reaction is distilled and separated. So that it is not in contact with the acidic potassium dichromate solution.
Aldehydes and Ketones
- 1. Reaction with HCN
- 2. Reaction with NaHSO3
- 3. Reaction with R-Mg-X
- 4. Reaction with Alcohol
Method: In a round-bottomed flask, take about 40 grams of potassium dichromate and about 160 ml of water. Put a two-hole cork in the flask. Put a separate funnel in one hole and attach the other to the condenser.
Fill the ether in the receiver and place it in the freezing mixture. A mixture of about 60 ml of ethyl alcohol and about 40 ml of concentrated sulfuric acid is taken into the funnel. Put the flask on the sand bath and heat it slowly.
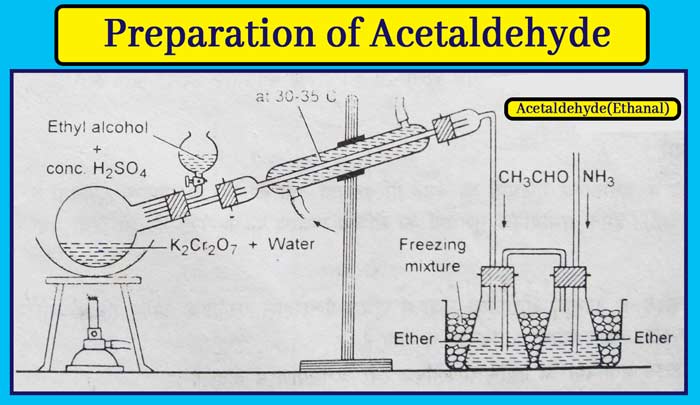
From the separating funnel, a mixture of ethyl alcohol and concentrated sulfuric acid drops by drop into the flask. Hot water flows up to 30-35°C in the condenser by which the high boiling ethyl alcohol and water vapors condensate and return to the flask in liquid form.
The vapors of acetaldehyde do not get condensate at this temperature and dissolve in the ether placed in the receiver. This ether solution also contains some amount of ethyl alcohol and water.
To obtain acetaldehyde in pure form, its ether solution is saturated with ammonia. The reaction of acetaldehyde and ammonia results in the formation of acetaldehyde ammonia which is a crystal solid. Pure acetaldehyde is obtained by filtering the crystals and distillation by mixing them with sulphuric acid.
Industrial Method
Following are the major methods of the Industrial method of acetaldehyde.
Acetylene: acetaldehyde is obtained when the acetylene gas is heated and diluted in the presence of mercuric sulfate in dilute H2SO4.
CH ≡CH + H2O → CH3CHO
In the presence of potassium hydroxide in methyl alcohol, the first methyl vinyl ether is obtained when Acetylene gas flows at 15 atmospheric pressure. Acetaldehyde is obtained by diluting this ether with dilute sulfuric acid.
CH ≡CH + CH3OH → CH3 – O – CH = CH2
CH3 – O – CH = CH2 → CH3CHO + CH3OH
Ethyl alcohol: Acetaldehyde is obtained by mixing a mixture of vapors and air of ethyl alcohol on a heated copper at a temperature of about 300°C.
2CH3CH2OH + O2 → 2CH3CHO + 2H2O
Physical Properties
It is a colorless, volatile and pungent liquid. Its boiling point is 21°C. It is miscible in water ethanol and ether.
Chemical Properties
Please check following Post for Chemical Properties of Acetaldehyde:
- How will you distinguish between aldehyde and ketone?
- Aldehydes and Ketones: Preparation, Properties, Nomenclature
Acetaldehyde is used in the manufacture of substances like ethyl alcohol, acetic acid, ethyl acetate, butyl alcohol, para and meta aldehyde etc. in industrial quantities.
Acetaldehyde-ammonia is used in the manufacture of synthetic rubber.
It is used in the manufacture of synthetic dyes.
Pata-aldehyde is used in making sleep medicine.
Meta-aldehyde is used as solid fuel in the sprit lamp.
- What is Acetaldehyde made of? | Properties, uses, and Tests
- How to test for Formaldehyde? Preparation, Properties and uses
- How will you distinguish between aldehyde and ketone?
- Aldehydes and Ketones: Preparation, Properties, Nomenclature
- Benzoic Acid: Formula, Structure, Properties, Uses, and Tests
- Benzaldehyde Formula, Preparation, Properties, uses, and Tests
- What is phenol used for? Preparation, Properties, uses, and Tests
Acetaldehyde gives all the normal tests of the aldehyde group. It gives pink color with schiff reagent. It makes a silver mirror with tollens reagent and gives a red precipitate with the fehling solution.
Specific testing of acetaldehyde is as follows.
- When heated with concentrated sodium hydroxide it gives off an unpleasant smell like dead bedbug.
- Alkaline sodium gives red color with nitroprusside.
- Paints blue with piperidine and sodium nitroprusside.
- When heated with iodine and NaOH or NH4OH, the molten crystals of Iodoform form.