Acetic Acid: What is acetic acid made of? | Properties | Uses
Chemical Formula
- Pyroligneous Acid – C5H4O2
- Acetamide – CH3CONH2
- Sodium Methoxide – CH3ONa
- Methyl Cyanide – CH3CN
- Silicon Dioxide – SiO2
- Hydrazoic Acid – HN3
Acetic Acid has been known since ancient times as vinegar. About 10% of vinegar is acetic acid. It was first built in 1720 by Stahl in an impure form. It was first obtained in pure form by Lavosier in 1786 by oxidation of ethyl alcohol.
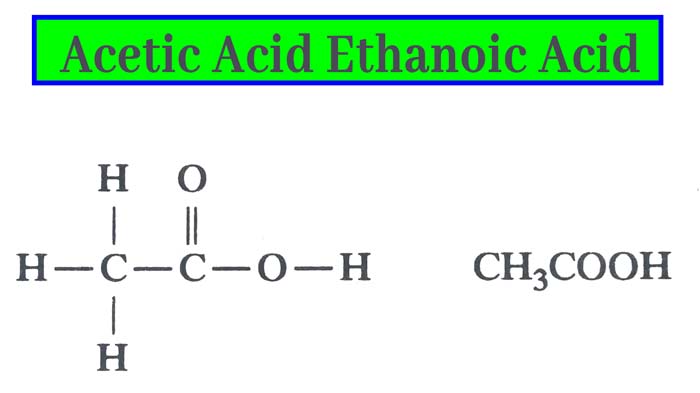
In 1845, Kolbe synthesized it from its elements. Its molecule formula C2H4O2 and structure formula are as below.
Industrial Preparation of Acetic Acid
The following are the major methods of industrial manufacture of acetic acid.
Synthesize Acetic Acid from Acetylene
In this method, firstly acetylene gas are obtained by the action of water with calcium carbide.
After this, acetaldehyde is obtained by flowing acetylene gas in a mixture of 42% sulfuric acid and 1% mercuric sulfate at 60°C heat. Thus obtained acetaldehyde is mixed with air vapours and flowing at high pressure and 70°C temperature with catalyst (vanadium pent oxide or manganese acetate) and the result we get oxidation of acetaldehyde. It gives Acetic Acid.
CaC2 + 2H2O → C2H2 + Ca(OH)2
C2H2 + H2O → CH3CHO
2CH3CHO + O2 → 2CH3COOH
The Determination of Acetic Acid in Pyroligneous Acid
Pyroligneous acid obtained from distillation of wood contains about 10% acetic acid in addition to water, 2-4% methyl alcohol and about 0.5% acetone.
To obtain acetic acid from pyroligneous acid, it is heated in a copper vessel. The vapours obtained from it flow into the milk of lime.
The vapour of acetic acid obtained by heating pyroligneous acid reacts with milk of lime to form calcium acetate. Due to being non-volatile, calcium acetate remains in the milk of lime.
The impurities of methyl alcohol, acetone etc. are dissociated as vapors. Calcium acetate is filtered and separated. After drying it is distilled with concentrated sulphuric acid to obtain acetic acid of about 75% concentration.
In order to obtain pure and anhydrous acetic acid from an aqueous solution of acetic acid, sodium hydroxide is first neutralized by aqueous solution of acetic acid.
When this solution evaporates, solid sodium acetate is obtained. Crystals from its aqueous solution yield crystals of sodium acetate. After distillation of these crystals with concentrated sulfuric acid, acetic acid of about 99% purity is obtained.
A small amount of water is also present in it. To remove small amounts of water, cool it to 16. On doing this, acetic acid is converted into a solid state.
Water remains in a fluid state. Small amounts of water are filtered or separated by any other suitable method. In this way pure and anhydrous acetic acid is obtained.
Pure and anhydrous acetic acid is obtained in the solid state similar to ice. Hence, it is called glacial acetic acid.
Because there is a substantial difference in the boiling point of water and the boiling point of acetic acid.
Hence, the efficient distillation method can also be used to obtain glacial acetic acid from aqueous solutions of acetic acid.
Nowadays due to the advancement of efficient distillation methods, glacial acetic acid is mainly made from this method by the aqueous solution of acetic acid.
Acetic Acid by Fermentation
Nowadays acetic acid is made in commercial quantities mainly from vinegar. In addition to water, vinegar contains about 6% Acetic acid, some other organic acids, some ester and some other substances.
Pure and anhydrous acetic acid can be made from the effective distillation of vinegar.
In India, vinegar is made by fermentation of sugarcane juice. In other countries vinegar is made by fermentation of ethyl alcohol.
When an aqueous solution of ethyl alcohol is left open in the air, it ferments and aqueous solution of acetic acid (vinegar) is obtained. Bacteria called mycoderma aceti(Mother of vinegar) are present in the air, which reach an aqueous solution of ethyl alcohol for their growth and convert it to vinegar by fermentation.
C2H5OH + O2 → CH3COOH + H2O
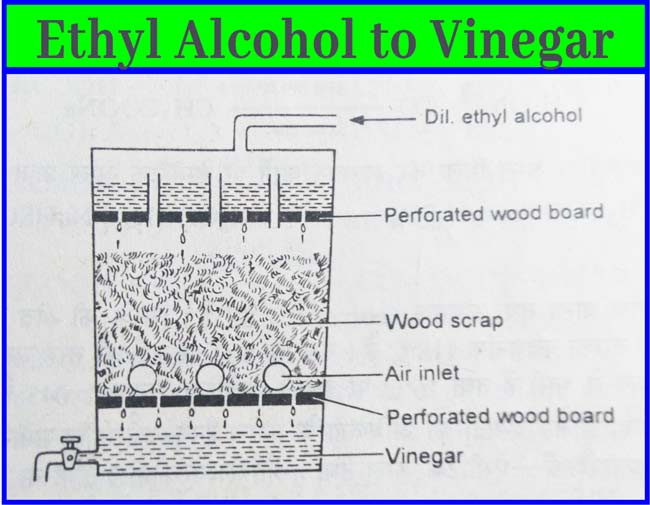
The method of making vinegar from ethyl alcohol is called quick vinegar method. In this method, a large keg of wood is used. This casks have wooden planks at the top and bottom. In between these two planks fill the clipping of vinegar-soaked beach wood. Some holes remain in the casks walls so that the fluid present in the casks remains in contact with air.
Approximately 10% aqueous solution of ethyl alcohol is slowly added upward in the casks. The temperature of the casks is kept at about 30-35°C. The fluid obtained from the bottom of the keg is slowly poured over it from time to time. After about 1 week vinegar is obtained from the lower part of the keg(casks).
Preparation of Acetic Acid by other method
Other major methods of making acetic acid –
Ethyl Alcohol
Acetic acid is obtained by oxidation of the acidic potassium dichromate solution of ethyl alcohol.
C2H5OH → CH3CHO → CH3COOH
Methyl Cyanide
Acetic acid is obtained from water decomposition by dilute acid of methyl cyanide.
CH3CN + 2H2O + HCl → CH3COOH + NH4Cl
Ethyl Acetate
Acetic acid is obtained from water decomposition by dilute acid of ethyl acetate.
CH3COOC2H5 + H2O → CH3COOH + C2H5OH
Acetamide
Acetic acid is obtained by the action of nitrous acid on Acetamide.
CH3CONH2 + HNO2 → CH3COOH + N2 + H2O
Acetic acid is also obtained from the water decomposition of acetamide.
Sodium Methoxide
Sodium acetate is obtained by passing carbon mono oxide at 200° temperature and 8 atmospheric pressure on sodium methoxide.
CH3ONa + CO → CH3COONa
Acetic acid is obtained after distillation by adding sulfuric acid to sodium acetate.
CH3COONa + H2SO4 → CH5COOH + NaHSO4
Physical Properties of Acetic Acid
It is a colorless, strong-smelling and corrosive liquid. The intense smell of vinegar is due to the presence of acetic acid in it. Its boiling point is 118°C. It freezes like snow at 16.5°C. It is soluble in water, alcohol and ether. It is heavier than water and its relative density is 1.045 at 16°C.
Due to the excess of hydrogen bonding in carboxyl group, their boiling points are higher than the boiling point of alcohol with the same molecular weight. Example: Both acetic acid and n propyl alcohol have molecular weights of 60, while acetic acid has a boiling point of 118°C and n – propyl alcohol has a boiling point of 97°C.
Chemical Properties of Acetic Acid
A molecule of acetic acid contains a methyl group and a carboxyl group. Hence, it exhibits reactions of methyl and carboxyl group. Due to the presence of methyl group, it exhibits only the halogenation reaction.
The carboxyl group participates in all its remaining reactions. These reactions of it represent the normal reactions of the carboxyl group. It does not have a formyl group present. Therefore, like formic acid, it does not exhibit detrimental properties.
Following are its major chemical reactions.
Reaction with Bases: acetic acid is a weak monobasic acid. And ionizes in aqueous solutions, forming cations of acetic ion (CH3COO–) and hydrogen. Due to its acidic properties it reacts with bases to form salts.
CH3COOH + NaOH → CH3COONa + H2O
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
CH3COOH + NH3 → CH3COONH4
Acetamide(CH3CONH2) is obtained by heating ammonium acetate(CH3COONH4).
CH3COONH4 → CH3CONH2
+ H2O
Carboxylic Acid
1. Introduction Carboxylic Acid
2. Nomenclature Carboxylic Acid
3. Structure Carboxylic Acid
Esterification: Just as the acid and base reacts to form
salts and water, similarly the reaction of acid and alcohol makes ester
and water. This reaction is called esterification. The esterification
action of carboxylic acid is often carried out in the presence of
concentrated sulfuric acid. It works as both anhydrous and catalyst.
<iframe width="834" height="469" src="https://www.youtube.com/embed/pyD2ZMby5Z4" title="Carboxylic Acid #12| Introduction| Nomenclature|| Structure|| Chemistry Page" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share" referrerpolicy="strict-origin-when-cross-origin" allowfullscreen></iframe>
CH3COOH + C2H5OH → CH3COOC2H5 + H2O
Halogenation: The hydrogen atoms of the methyl radical are displaced one by one from the chlorine or bromine atoms when chlorin or bromin is flowed in acetic acid in the presence of red phosphorus.
CH3COOH + Cl2 → CH2ClCOOH + HCl
CH2ClCOOH + Cl2 → CHCl2COOH + HCl
CHCl2COOH + Cl2 → CCl3COOH + HCl
Similar reaction with chlorin and bromin occurs in the presence of red phosphorus of other carboxylic acid. In this reaction the hydrogen atoms present on the adjacent carbon atom of the carboxylic acid group i.e. α-hydrogen atom are displaced by the halogen atoms. This reaction is called Hell Volhard Zelinsky reaction (HVZ Reaction).
Reaction with PCl5: It reacts with PCl5 to form acetyl chloride (CH3COCl). In this reaction, the -OH group of the -COOH group is displaced from the chlorine atom.
CH3COOH + PCl5 → CH3COCl + POCl3 + HCl
Same reaction is also occur with PCl3 and SOCl2.
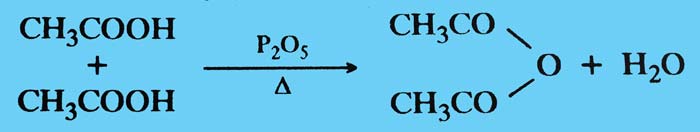
Dehydration: When heated acetic acid alone or in the presence of anhydrous agents such as phosphorus pentoxide, one of its two molecules dissociates and produces acetic anhydrid.
Reduction: Lithium is reduced by aluminum hydride to form ethanol.
CH3COOH – LiAlH4 → CH3CH2OH
It can also be reduced by H2 / Ni, Na / alcohol and NaBH4.
Reactions to salts: Methane is obtained by heating sodium acetate with soda lime.
CH3COONa + NaOH → CH4 + Na2CO3
Ethane is obtained after the legal decomposition of sodium or potassium acetate.
2CH3COONa + 2H2O → C2H6 + 2CO2 + 2NaOH + H2
Acetone is obtained by heating calcium acetate alone.
(CH3COO)2Ca → CH3COCH3 + CaCO3
Acetaldihyde is obtained by mixing calcium phormate with calcium acetate.
(CH3COO)2Ca + (HCOO)2Ca → 2CH3COCH3 + CaCO3
Methyl bromide is obtained by reacting with bromine in a dry CCl4 solution of silver salts of acetic acid. This reaction is called Hunsdiecker Reaction.
CH3COOAg + Br2 → CH3Br + CO2 + AgBr
Schmidt Reaction: It reacts with hydrejoic acid in the presence of concentrated sulfuric acid to form methyl amin. This reaction is called Schmidt Reaction.
CH3COOH + N3H → CH3NH2 + CO2 + N2
Acetic Acid Uses
As reagent in the laboratory
Used in making acetone and esters, which are used in making Perfume dyes plastic and medicine.
In the manufacture of artificial fibers called cellulose acetate and polyvinyl acetate.
making white and artificial vinegar
which is an oxidizing agent in making tetra acetate.
Rubber quivering
Acetic Acid Tests
Acetic acid and acetate ion are tested by the following methods –
Acetic acid has a vinegar-like odor. Vinegar smells when heated with concentrated sulfuric acid.
In a neutral solution of acetic acid, the color of the solution turns red by adding a few drops of the neutral solution to the ferric chloride.
On heating with a few drops of ethyl alcohol and concentrated sulfuric acid, ethyl acetate is formed which has a sweet smell like fruit.
The solutions of acetic acid and its salts do not show the reducing properties. Hence, acetic acid does not reduce ammonia silver nitrate solution or Fehling solution.
Cacodyl Test: Upon mixing and evaporating arsenious acid in an acetic acid solution neutralized by potassium dioxide, a substance called Cacodyl oxide [(CH3)4As]O2 is formed which has a strong odor.
In addition to the above tests, acetic acid also gives a general test of carboxylic acid (NaHCO3). Effervescences are obtained due to the formation of CO2 gas upon the addition of NaHCO3 solution to acetic acid.
