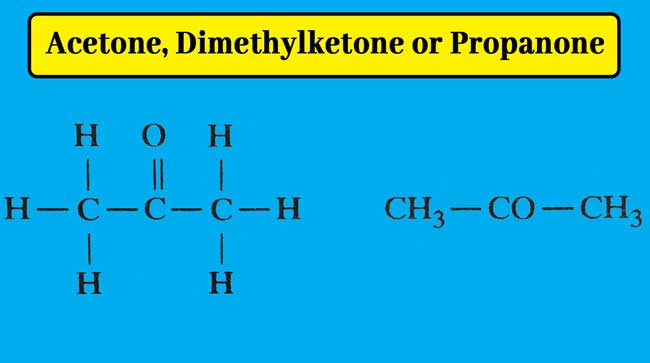
What is acetone used for? | Preparation, Properties, Uses, and Tests
Acetone is the first member of ketones. Its molecule formula C3H6O and structure formula are following.
 Preparation
Preparation
Laboratory Method
In the laboratory acetone is obtained by dry distillation of calcium acetate.
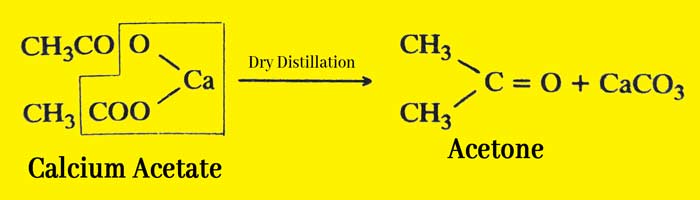
Method: Take powdered calcium acetate in a metal ritard or hardened glass flask. They organize the condenser and receiver. Carefully heat the flask by placing it on a wire mesh. Acetone is distilled and collected in the receptor.
For the treatment of acetone obtained in this method, by adding sodium bisulfite to it, it is shaken a lot, by which the crystals of acetone sodium bisulfite are obtained.
These crystals are filtered and separated. By distillation of these crystals in a saturated solution of sodium carbonate, acetone is obtained in the receptor which also contains some amount of water.
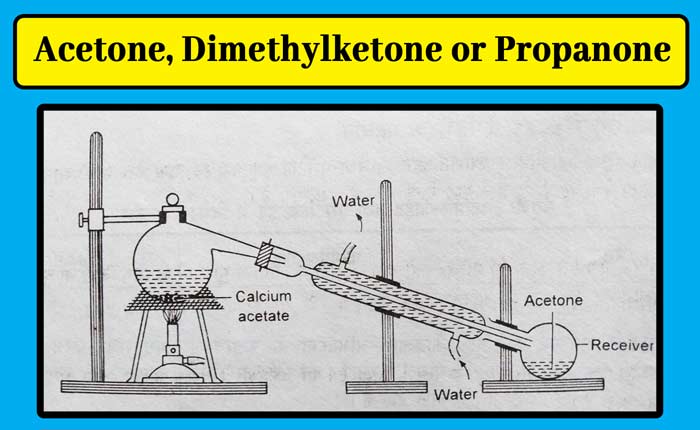
Anhydrous calcium chloride is added to this mixture to remove small amounts of water. The calcium chloride is filtered and separated and distillation of the remaining fluid gives pure acetone.
Industrial Method
The following is the key to the industrial manufacture of acetone.
Propylene
Iso propyl hydrogen sulfate is formed by the flow of propylene obtained from the bleaching of petroleum into concentrated sulfuric acid.
Iso propyl alcohol is obtained as a result of water decomposition when heated with dilute sulfuric acid. Thus Acetone is obtained by passing the vapors of iso propyl alcohol obtained at 300°C over heated copper.
CH3 – CH = CH2 + H2SO4 → CH3.CH(HSO4).CH3
CH3.CH(HSO4).CH3 + H2O → CH3.CH(OH).CH3 + H2SO4
CH3.CH(OH).CH3 → CH3COCH3 + H2
Acetic Acid
Acetone is obtained when vapors of acetic acid are precipitated on heated alumina (Al2O3) or magnesium oxide at about 300-350°C.
CH3COOH + CH3COOH → CH3COCH3 + H2O + CO2
Acetone is obtained when vapors of acetic acid are precipitated on heated alumina (Al2O3) or magnesium oxide at about 300-350°C.
- What is acetone used for? | Preparation, Properties, Uses, and Tests
- What is Acetaldehyde made of? | Properties, uses, and Tests
- How to test for Formaldehyde? Preparation, Properties and uses
- How will you distinguish between aldehyde and ketone?
- Aldehydes and Ketones: Preparation, Properties, Nomenclature
- Benzoic Acid: Formula, Structure, Properties, Uses, and Tests
- Benzaldehyde Formula, Preparation, Properties, uses, and Tests
- What is phenol used for? Preparation, Properties, uses, and Tests
Wood Distillation
Pyroligneous acid obtained from distillation of wood contains acetic acid, acetone and methyl alcohol. The vapors obtained by heating Pyroligneous acid flow into the hot water.
The vapor of acetic acid reacts with the selected water to form calcium acetate. The vapors of methyl alcohol and acetone are liquefied and collected in the receptor.
Acetone is obtained by dry distillation of calcium acetate. Acetone is also obtained from the effective distillation of a mixture of methyl alcohol and acetone.
Physical Properties
Acetone is a colorless liquid. It has a distinctive and sweet smell. It is a volatile and flammable liquid. Its boiling point is 56°C. It is soluble in ether, water and alcohol. Propanone is the IUPAC name of Acetone.
Chemical Properties
Please check following Post for Chemical Properties of Acetone:
- How will you distinguish between aldehyde and ketone?
- Aldehydes and Ketones: Preparation, Properties, Nomenclature
- acetone is used as a good solvent. It is used to dissolve washing, cellulose, artificial silk, cordite etc.
- Sulphonal, chloretone, chloroform, iodoform etc. useful substances used to make.
- It is also used in making artificial perfumes and synthesized rubber.
Specific tests of acetone are as follows.
Iodoform Test: Yellow crystals of iodoform are obtained by heating acetone with iodine and sodium hydroxide or ammonium hydroxide. The iodoform is also obtained by the reaction of ethyl alcohol with iodine and sodium hydroxide but the iodoform is not obtained by the reaction of ethyl alcohol with iodine and ammonium hydroxide.
Legal test: On adding a few drops of sodium hydroxide and fresh Sodium nitroprusside solution to acetone, orange color is obtained which turns yellow after some time. Adding ammonium hydroxide in place of sodium hydroxide gives a violet color.