— Halogenation:

— Friedel-Crafts reaction:

— Nitration:

The following important topic list in chapter.
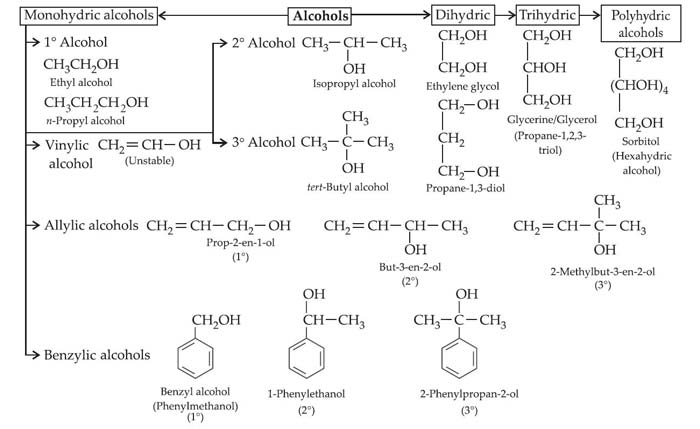
General formula: Alcohols are the hydroxyl derivatives of alkanes having general formula CnH2n+1OH.
Nomenclature: In a common system, alcohol is named as alkyl alcohol. According to the IUPAC system, alcohols are called ‘alkanols’, by replacing ‘-e’ of alkane by ‘-ol’.
Structure: In alcohols, R OH, the O atom of the hydroxyl group is attached to C-atom by a sigma (σ) bond formed by the overlap of sp3 hybridized orbital of C-atom with sp3 hybridized orbital of O atom.
Classification: Alcohols are classified as monohydric, dihydric, trihydric and polyhydric alcohols depending upon the number of Hydroxyl (—OH) groups present in the molecule.
X From alkenes:
— By acid-catalyzed hydration:

— By hydroboration-oxidation:

From carbonyl compounds:
— By reduction of aldehydes and ketones:

— By reduction of carboxylic acids and esters:
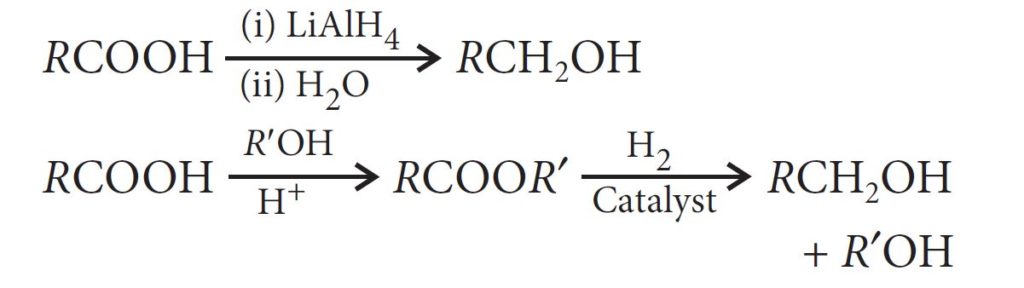
— From Grignard reagents:


Physical state: Lower alcohols are colorless liquids with characteristic smell while higher alcohols are colorless, odorless waxy solids.
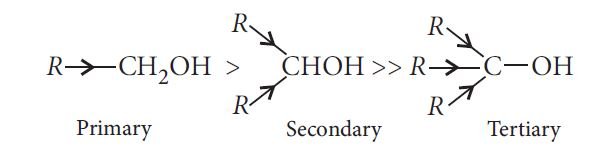
Solubility: Solubility of alcohol in water is due to their ability to form hydrogen bonds with water molecules. The solubility decreases with an increase in the size of alkyl groups and solubility increases with an increase in branching and the order is 1° < 2° < 3°.
Boiling points: The boiling points of alcohols increase with increase in the number of carbon atoms as van der Waals forces increase and the boiling points decrease with increase of branching in carbon chain due to decrease in van der Waals forces with decrease in surface area and the order is 1° > 2 ° > 3°.
Chemical Properties: Alcohols react both as nucleophiles (when the bond between O H is broken) and electrophiles (when the bond between C O is broken).
Reactions involving cleavage of O—H bond:
— The acidity of alcohols (reaction with metals):

— The acid strength of alcohols decreases in the following order:
— Esterification:
ROH + (R′CO)2O or R′COCl → RCOOR′
X Reactions involving cleavage of C—O bond:
— Reaction with hydrogen halides:
ROH + HX RX + H2O
— Lucas test: Lucas reagent is a solution of conc. HCl with anhyd. ZnCl2.
— With Lucas reagent,
Primary alcohols – No cloudiness
Secondary alcohols – Cloudiness in 5 minutes
Tertiary alcohols – Cloudiness immediately
— Reaction with phosphorus trihalides:
ROH + PCl3 → RCl + H3PO3
— Dehydration:
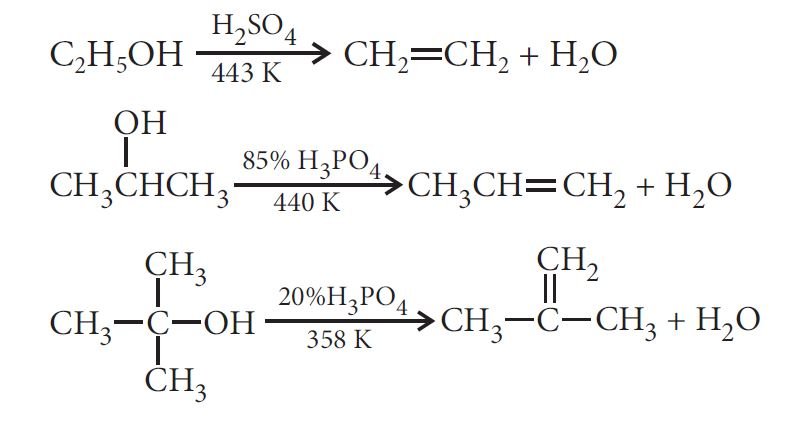
Thus, the relative ease of dehydration of alcohols follows the following order:
Tertiary > Secondary > Primary
— Oxidation:
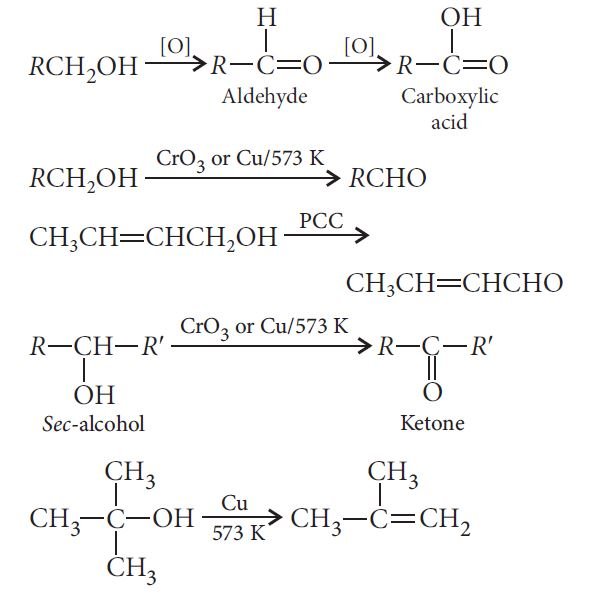
Some commercially important alcohols:
Methanol (wood spirit): It is produced by catalytic hydrogenation of CO in the presence of ZnO – Cr2O3 as the catalyst at high temperature and pressure.
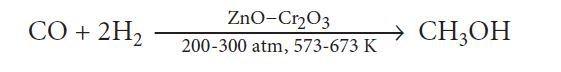
— Methanol is a colorless liquid and is highly poisonous in nature.
— Ingestion of small quantities can cause blindness while large quantities can cause death.
— It is used as a solvent in paints, varnishes and for making formaldehyde.
Ethanol: It is commercially manufactured by using fermentation of sugar present in molasses, sugarcane or fruits such as grapes.
Formula: C2H5OH
— Ethanol is a colourless liquid and is used as a solvent in the paint industry and in preparation for a number of carbon compounds.
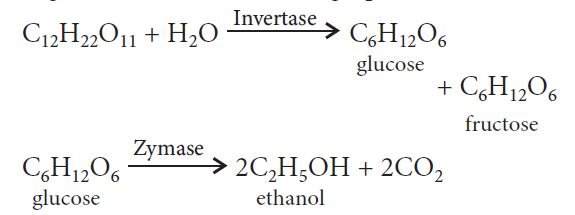
— Commercial alcohol made unfit for drinking by mixing it with copper sulfate and pyridine is called denatured alcohol.
PHENOLS
General formula: Phenols are the compounds in which the hydroxy ( OH) group is directly linked to the aromatic ring having formula C6H5OH.
Nomenclature: The simplest hydroxy derivative of benzene is phenol also called carbolic acid. It is its common name and also an accepted IUPAC name.
Structure: In phenols, the —OH group is attached to sp2 hybridised C-atom of an aromatic ring.
Classification: Like alcohols, phenols are also classified as mono and polyhydric phenols.
Preparation:
From Haloarenes:

From benzene sulphonic acid:
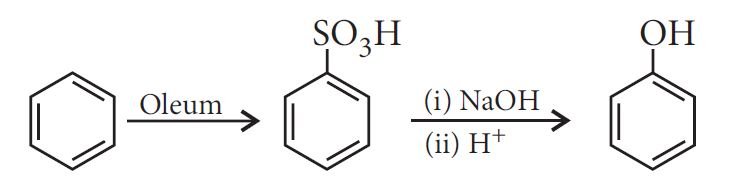
From diazonium salts:
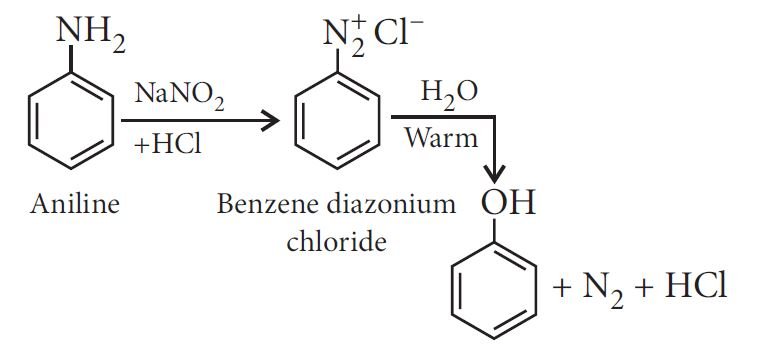
From cumene:

Physical state: Phenols are colorless crystalline solids or liquids.
Odour: They have characteristic phenolic odours.
Solubility: Like alcohol, phenols are soluble in water due to the formation of hydrogen bonding with water.
— Phenols are less soluble than alcohol due to large hydrocarbon (benzene ring) part.
— Phenols are soluble in alcohols, ethers and also in NaOH.
Boiling points: Much higher than the corresponding aromatic hydrocarbons and haloarenes due to intermolecular hydrogen bonding.
The acidity of phenols: Phenols are weakly acidic in nature due to polar O H bond directly attached to sp2-hybridized C-atom.
— They turn blue litmus red and react with alkali metals and alkalies to form their salts.
— Phenol is a weaker acid than a carboxylic acid. It does not react with Sodium Carbonate (Na2CO3) and sodium bicarbonate (NaHCO3).
— Phenols are more acidic than alcohol which can be explained on the basis of resonance.
— Electron withdrawing groups increase the acidic strength of phenols.
— Electron releasing groups decrease the acidic strength of phenols.
— Reaction with metals:
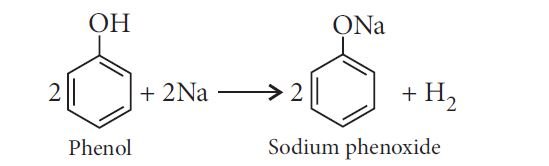
In addition to this, phenols react with aqueous sodium hydroxide to form sodium phenoxides.

Electrophilic aromatic substitution:
— Nitration:

— Halogenation:

Kolbe’s reaction:
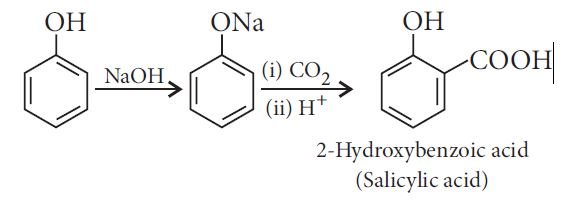
Reimer-Tiemann reaction:
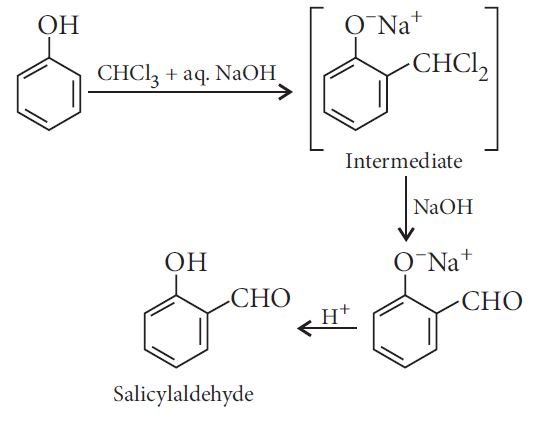
The reaction of phenol with zinc dust:

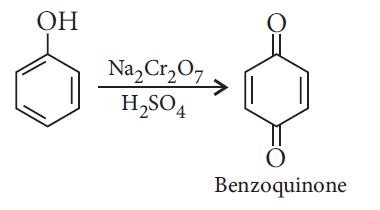
Oxidation
— Ferric chloride test: Phenol gives violet color with a neutral FeCl3 solution.

— Bromine water test: Phenol gives white ppt. with Br2-water due to the formation of 2, 4, 6-tribromophenol.

General formula: Ethers are the compounds having general formula CnH2n+2O where n is always greater than 1.
Nomenclature: Common names of Ether are derived from the names of alkyl/aryl groups written as separate words in alphabetical order and adding the word ‘ether’ at the end. If both the alkyl groups are the same, the prefix ‘di’ is added before the alkyl group.
According to the IUPAC system of nomenclature, Ethers are regarded as hydrocarbon derivatives in which a hydrogen atom is replaced by an OR or OAr group, where R and Ar represent alkyl and aryl groups, respectively. The larger R group is chosen as the parent hydrocarbon.
Structure: In ethers, the four-electron pairs, i.e., the two bond pairs and two lone pairs of electrons on O atom are arranged approximately in a tetrahedral arrangement.
Classification: Ethers can be classified as symmetrical or simple ethers having formula, R — O — R and unsymmetrical or mixed ethers having the formula, R — O — R′.
From alcohols by dehydration:
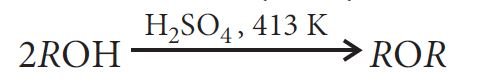
From alkyl halide:
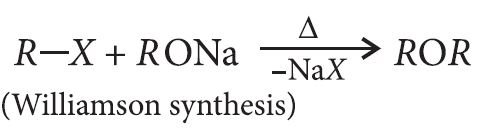
— Williamson synthesis can be used to prepare ethers containing 2° or 3° alkyl groups through SN2 mechanism. In this case, the alkyl halide must be 1°. In the case of 2° and 3° alkyl halides, elimination takes place. It cannot be used to prepare diaryl ethers.
— Dehydration of alcohols for the formation of ethers follows the order: 1° > 2° > 3°
Physical state and odour: Dimethyl ether and ethyl methyl ether are exceptionally gases at room temperature while all other ethers are colorless liquids with the characteristic ethereal smell.
Solubility: Ethers are soluble in water to a certain extent due to hydrogen bonding.
— Solubility decreases with increase in molecular mass.
— Ethers are fairly soluble in all organic solvents such as alcohol, chloroform, benzene, etc.
Boiling points: Ethers have lower boiling points than isomeric alcohols due to their inability to form hydrogen bonds and get associated.
— But lower ethers have slightly higher boiling points than n-alkanes of comparable molecular masses due to dipole-dipole interactions.
— Higher ethers (containing carbon atom more than four) have slightly lower boiling points than n-alkanes of comparable molecular masses due to weak van der Waals forces of attraction.
— Polarity: Ethers are polar in nature.
— Density: Ethers have low density. All Ethers are lighter than water.
Cleavage of C—O bond in ethers:
ROR + HX RX + ROH
ROH + HX R X + H2O
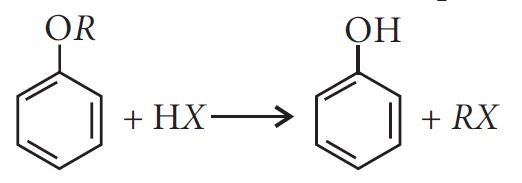
ROR′ + HX RX + R′OH
The order of reactivity of hydrogen halides is as follows: HI > HBr > HCl.

— Halogenation:

— Friedel-Crafts reaction:

— Nitration:
