How will you distinguish between aldehyde and ketone?
Aldehydes and Ketones
Common tests of Aldehydes and Ketones
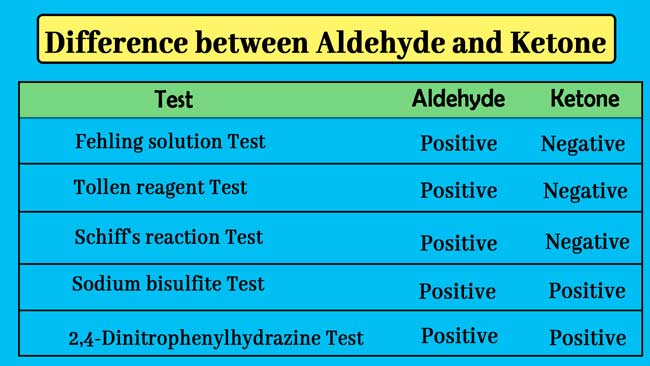
Differentiation between aldehyde and ketone and other compounds can be identified based on general tests of aldehydes and ketones. The following are common tests of aldehyde and ketone.
01. 2,4-Dinitrophenylhydrazine test: If a yellow, orange or red precipitate is obtained when heating the carbonic compound with 2,4-Dinitrophenylhydrazine solution in the presence of concentrated sulfuric acid, it exhibits the presence of aldehyde or ketone group.
02. Sodium bisulfite test: If a white precipitate is obtained when the carbonic compound is shaken with a saturated solution of sodium bisulfite, it exhibits the presence of aldehyde or ketone group.
03. Schiff‘s Reaction Test: If the carbonic compound is added to the schiff reagent, it exhibits a pink color, indicating the presence of aldehyde group.
04. Tollen reagent test: shows the presence of aldehyde group if the carbonic compound is obtained with a silver mirror when heated with tollen reagent.
05. Fehling solution test: If the carbonic compound gets red color on heating with fehling solution then it shows the presence of aldehyde group.
If a compound gives positive results in all the above tests then it is an aldehyde. If a compound gives positive results in Test no. 1 and 2 and negative in Test no. 3, 4 and 5, then it is a ketone.
Aldehyde Reactions
Polymerization of Aldehydes
The first few members of the aldehyde series exhibit polymerization reactions.
Example:
(i) Aqueous solution of formaldehyde evaporates to yield a white solid substance called para formaldehyde.
n HCHO → (HCHO)n
The value of n ranges from 6 to 100 in the above reaction. The melting point of para formaldehyde is 121°-123°C. By heating it, formaldehyde can be obtained again.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
-
Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
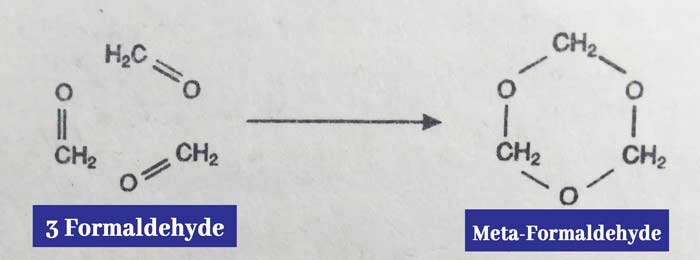
(ii) When formaldehyde gas is kept at room temperature for a long time, it polymerise to form meta formaldehyde.
(iii) By pouring a few drops of concentrated H2SO4 at an ordinary temperature in anhydrous acetaldehyde, three molecules of acetaldehyde combine to form para aldehyde.
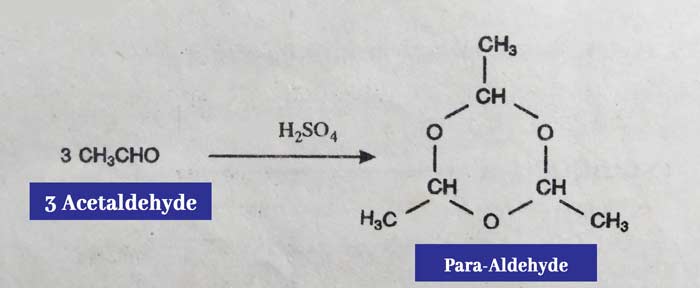
Re-acetaldehyde can be obtained by distilling para aldehyde with dilute H2SO4.
(iv) After adding a few drops of concentrated H2SO4 at 0°C to anhydrous acetaldehyde, its four molecules combine to form meta aldehyde.

Schiff‘s Reaction
Rosaniline hydrochloride, also called megenta, is pink in color. The color of the solution flies away when SO2 gas flows into its aqueous solution. This colorless solution is called schiff reagent. The schiff reagent is reaction with aldehyde and gives pink colour.
Reaction with sodium hydroxide
Aldehydes without α-hydrogen such as formaldehyde when heated with concentrated NaOH results in their disproportionation. An alcohol and salts of a fatty acid are obtained as a result of this reaction.
Example:
2HCHO + NaOH → CH3OH + HCOONa
This reaction is called the Cannizzaros Reaction.
Aldehydes containing α-hydrogen reaction with Concentrated NaOH give complex regent-like substances. Ketone does not show these reactions.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
The reaction of formaldehyde and ammonia results in the formation of Hexamethylenetetramine [(CH2)6N4] which is also called urotropin.
6HCHO + 4NH3 → (CH2)6N4 + 6H2O
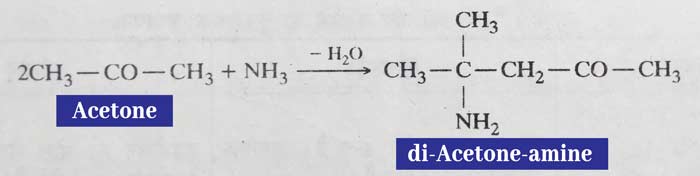
Other aldehyde forms additive products with ammonia, which are called aldehyde ammonia.
Example:
Reducing Properties
aldehyde is a strong reducing agent, hence reducing tollens reagent and fehling solution. Ketone does not reduce these reagents.
Tollen’s Reagent | Fehling & Benedict’s Solution
1. Oxidation of aldehydes a) Oxidation by Tollen’s reagent b) Oxidation by Fehling solution c) Oxidation by Benedict’s solution 2. Oxidation of ketones a) By strong oxidizing agents b) Popoff’s rule
<iframe width="834" height="469" src="https://www.youtube.com/embed/FG1gSgfi6a4" title="Oxidation of Aldehyde and Ketones #7| Tollen’s Reagent | Fehling & Benedict’s Solution|Popoff’s rule" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share" referrerpolicy="strict-origin-when-cross-origin" allowfullscreen></iframe>
CH3CHO + Ag2O → CH3COOH + 2Ag
CH3CHO + 2CuO → CH3COOH + Cu2O
Ammonia silver nitrate solution is called tollens reagent. By reducing aldehyde tollens reagent, it forms metallic silver which is obtained in the form of silver mirror. On heating with fehling solution, they give red precipitates due to formation of Cu2O.
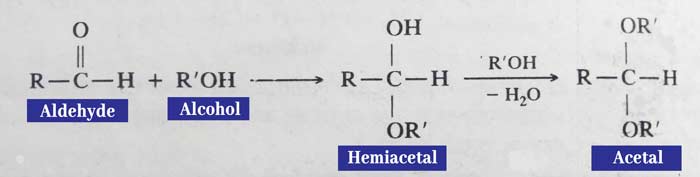
Reaction with Alcohol
In the presence of dry hydrogen chloride gas, the reaction of alcohols and aldehydes forms a temporary compound called hemiacetal which then reacts with alcohol to form a permanent compound called acetal.
Ketones reactions
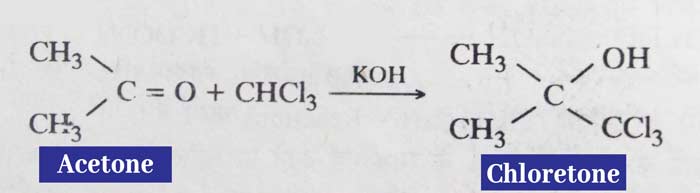
Reaction with chloroform
In the presence of caustic potash (KOH), chloroform reacts with kitones to form chloro hydroxy compounds.
Example:
Reaction with nitrous acid
Ketone reacts with nitrous acid to make oximino ketones.
example:
CH3 – CO – CH3 + O = N – OH → CH3COCH = NOH + H2O
Reaction with Sodamide
Ketone reacts with ether solution of sodium or sodamide to form sodium products. Example:
CH3COCH3 + NaNH2 → CH3 – CO – CH2Na + NH3