Amine Properties and Uses || What are the different types of amines?
Interpretation of amine: The product after the substitution of one or more hydrogen atoms in the ammonia molecule with a hydrocarbon group is called an amine. In this lesson, we will discuss Amine Properties and Uses.
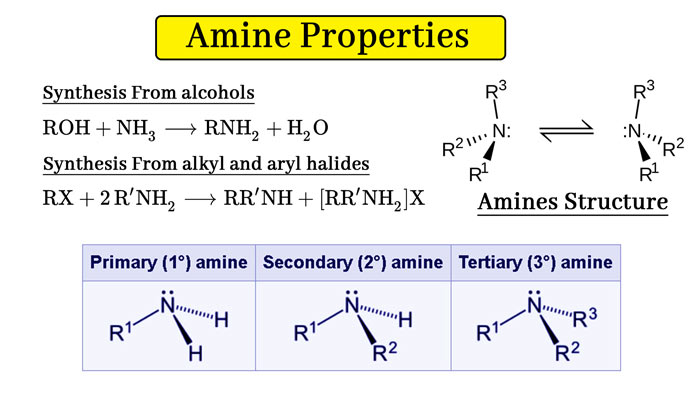
According to the number of hydrogen atoms substituted in the amine molecule, the amine can be divided into primary, secondary, and tertiary amines, ammonia Hydrogen in the molecule is replaced by a hydrocarbon group.

At the same time, the amine can be regarded as a derivative of H in the ammonia molecule replaced by a hydrocarbon group. Amines are widely present in the biological world and have extremely important physiological and biological activities, such as Protein, nucleic acids, many hormones, antibiotics, and alkaloids. They are all complex derivatives of amines. Most drugs used in clinical practice are also amines or amine derivatives.
Therefore, mastering the properties and synthetic methods of amines is to study these complex natural products and Basis for better maintenance of human health.
Classification of Amines
According to the number of hydrogen substituted, it is divided into:
- primary amine (primary amine) RNH2
- secondary amine (secondary amine) R2NH
- Tertiary amine (tertiary amine) R3N
Quaternary ammonium salt R4N+X – such as
- Methylamine CH3NH2
- Aniline C6H5NH2
- Ethylenediamine H2NCH2CH2NH2
- Diisopropylamine [(CH3)2CH]2NH
- Triethanolamine (HOCH2CH2)3N
- Tetrabutylammonium bromide (CH3CH2CH2CH2)4N+Br–.
The tetrahydroxy substitution of ammonium hydroxide or ammonium salt is called quaternary ammonium hydroxide or Quaternary ammonium salt.
NH4+ ammonium R4NOH quaternary ammonium base R4NX quaternary ammonium salt
Depending on the type of hydroxyl group attached to the nitrogen atom in the amine molecule. Amines can be divided into fatty amines and aromatic amines. If the amine molecule contains two or more amino groups (—NH2), they can be divided into diamines and triamines according to the number of amino groups.
Amines Properties :
Amines are organic compounds derived from ammonia (NH₃) where one or more hydrogen atoms are replaced by alkyl or aryl groups. They are categorized into primary, secondary, and tertiary amines based on the number of alkyl or aryl groups attached to the nitrogen atom. Here is a list of their key properties:
Basicity:
- Amines are basic in nature due to the lone pair of electrons on the nitrogen atom, which can accept a proton (H⁺).
- The basicity of amines can be influenced by the electronic effects of substituents. For example, alkyl groups generally increase basicity, while aryl groups decrease it.
Hydrogen Bonding:
- Primary and secondary amines can form hydrogen bonds due to the presence of N-H bonds, leading to higher boiling points and solubility in water compared to tertiary amines, which lack N-H bonds.
Boiling Points:
- Amines typically have lower boiling points than alcohols of comparable molecular weight due to the weaker hydrogen bonding.
- Primary and secondary amines have higher boiling points than tertiary amines due to hydrogen bonding.
Solubility:
- Low molecular weight amines are generally soluble in water because they can form hydrogen bonds with water molecules.
- Solubility decreases with increasing molecular weight and the presence of hydrophobic alkyl groups.
Odor:
- Many amines have a characteristic fishy odor.
- Lower amines tend to have more pungent smells, while higher amines have less intense odors.
Reactivity:
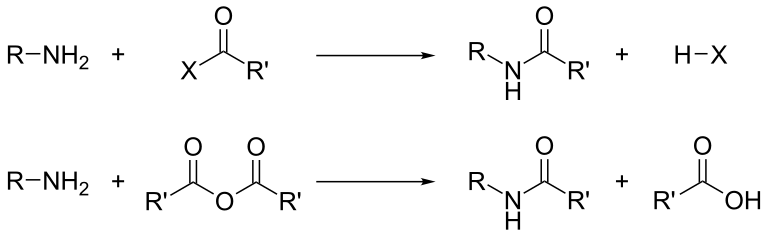
- Amines are nucleophilic and can participate in a variety of chemical reactions, including alkylation, acylation, and reactions with carbonyl compounds.
- They can form Schiff bases and amides and are also involved in the synthesis of many pharmaceuticals and dyes.
Acid-Base Reactions:
- Amines react with acids to form ammonium salts, which are typically more soluble in water than the parent amine.
- This property is used to separate amines from non-basic organic compounds in extraction processes.
Electronic Effects:
- The electron-donating nature of alkyl groups can increase the electron density on the nitrogen, enhancing the nucleophilicity and basicity of the amine.
- Conversely, electron-withdrawing groups (like aryl groups) decrease the electron density on the nitrogen, reducing the basicity.
Chirality:
- Amines can be chiral if the nitrogen atom is bonded to three different substituents and a lone pair. However, the inversion of configuration (nitrogen inversion) usually makes the resolution of chiral amines challenging.
Spectroscopic Properties:
- Amines exhibit characteristic peaks in infrared (IR) spectroscopy due to N-H stretching and bending vibrations.
- In nuclear magnetic resonance (NMR) spectroscopy, amines show distinct chemical shifts for hydrogen and carbon atoms adjacent to the nitrogen atom.
Formation of Complexes:
- Amines can act as ligands to form complexes with transition metals, often stabilizing various oxidation states of the metals.
Physical and Chemical Properties
At room temperature, lower fatty amines are gases, propylamines are liquids, and higher fatty amines are solids. Lower amines have an unpleasant or bad smell.
For example, trimethylamine has a fishy smell, but succinyl amine (putrescine) and glutamine (cadaverine) have a bad smell after the animal carcass rots.
Higher amines are less volatile and have a lower odor. Aromatic amines are high-boiling liquids or low-melting solids. Although they are less odorous than fatty amines, they are more toxic. They can cause poisoning whether they inhale their vapor or contact the skin. Some aromatic amines, such as β-naphthylamine, and benzidine also have carcinogenic effects.
Because the nitrogen atom in the amine molecule can form a hydrogen bond with water, the solubility of lower fatty amines in water is relatively large.
Primary and secondary amines can form intermolecular hydrogen bonds, but because the electronegativity of the nitrogen atom is less than that of the oxygen atom, the hydrogen bonding ability of the amine is relatively weak, and its boiling point is lower than that of alcohols with similar molecular weight.
Amine is similar to ammonia in that the nitrogen atom in the molecule contains an unshared electron pair, which can be combined with H + to be alkaline.
NH2-R & lt2 + NH2-R & lt ≒ HCI3 + CI –
The basicity of the amine is expressed by the basic ionization constant Kb or other negative logarithmic values pKb. The larger the Kb value or the smaller the pKb value, the stronger the basicity of the amine.
Synthesis of Amines
Ammonolysis of halides
There is a lone pair of electrons on the ammonia or amine nitrogen, which acts as a nucleophile and undergoes a nucleophilic reaction with a haloalkane. Many organic halides are treated with ammonia or ammonia solution to turn into amines:
X is halogen
NH2 + the RX3 → RNH23 + X- –
RNH23 + X- – → RNH22 + H2O + X- –
Made with alcohol
The main synthetic method of amines is the alkylation of ammonia. Industrial use of alcohols and ammonia to synthesize organic amines:
ROH + NH3 → RNH2 + H2O
These reactions require the use of catalysts, special equipment, and additional purification, because the mixture of primary, secondary, and tertiary amines is obtained, and the selectivity of the reaction needs to be improved.
Preparation of Amine
Amines are widely distributed in nature, most of which is produced by decarboxylation of amino acids.
Most of the industrial methods for preparing amines are obtained by the reaction of ammonia with alcohol or haloalkanes. The product is a mixture of amines at various levels, and the pure product is obtained after fractional distillation.
The corresponding amines can also be obtained by catalytic reduction of aldehydes and ketones in the presence of ammonia. In industry, amine compounds are often prepared by catalytic reduction of nitro compounds, nitriles, amides or nitrogen-containing heterocyclic compounds.
Application of Amines
Amine has a wide range of uses. The earliest development of the dye industry was based on aniline. Some amines are necessary for life support
Yes, but some are very harmful to life. Many amine compounds have carcinogenic effects, especially aromatic amines, such as naphthylamine and benzidine.
Structure of Amine
The structure of the nitrogen atom in the amine, much like the nitrogen atom in the ammonia molecule, is connected to hydrogen or a hydrocarbon group by three sp hybrid orbitals, forming a pyramid, leaving an sp3 hybrid orbital occupied by a lone electron pair. If an amine has three different groups, there should be a pair of enantiomers (see Enantiomers):
However, due to the low activation energy required for the lone electron pair in the inverted amine molecule, its enantiomers could not be separated.
The experiment proves that the amine and ammonia molecules have a pyramidal structure, the nitrogen atom is sp3 hybrid, and the bond angle is about 109 degrees.

In the amine molecule, the three sp3 hybrid orbitals overlap with the hydrogen atom’s s orbital or the carbon atom’s hybrid orbit to form three 6 bonds, and the remaining pair of lone pairs of electrons occupy the fourth sp3 orbit and are located in the prism. The top.
Aniline is also a pyramidal structure, but the HNH bond angle is large, 113.9 degrees, and the angle at which the NNH plane intersects the benzene ring plane is 38 degrees.
If three different groups are attached to the nitrogen atom in the amine molecule, it has chirality and theoretically, there should be a pair of enantiomers.
Because the energy barrier between the two enantiomers is quite low, about 21KJ / mol, they can be quickly converted to each other at room temperature. In fact, such enantiomers have not yet been separated.
In the quaternary ammonium salt, all four sp3 orbitals of nitrogen are used to form bonds. If four different groups are connected to the nitrogen atom, enantiomers exist.
For example, iodinated methyl dipropyl phenyl benzylamine is resolved into right and left optical isomers.
Several common amines
Methylamine: CH3NH2
Properties: Also known as monomethyl amine. It is a colorless ammonia odor gas or liquid at room temperature. It is easy to burn, and its vapor can form an explosive mixture with air, and the explosion limit is 5% -21% (4.95% -20.75%).
Relative density is 0.662, melting point is -93.5 ℃, boiling point is -6.3 ~ -6.7 ℃, -19.7 ℃ (53.3kPa), -32.4 ℃ (26.7kPa), -43.7 ℃ (13.3kPa), -73.8 ℃ (1.33kPa) , Decomposition temperature 250 ℃, flash point (closed cup)0℃, auto-ignition point 430 ℃, vapor pressure (25 ℃) 202.65Pa, critical temperature 156.9 ℃, critical pressure 4.073kPa, refractive index 1.351.
After liquefaction, the smoke body is more alkaline than ammonia. Soluble in water, ethanol, and ether.
Ethylamine: CH3CH2NH2
Colorless liquid or gas with strong ammonia smell, vapor pressure 53.32kPa / 20℃, flash point: <-17.8 ℃, melting point -80.9 ℃, boiling point 16.6 ℃, solubility: soluble in water, ethanol, ether, etc. Density: Relative density (water = 1) 0.70; Relative density (air = 1) 1.56. Stability: stable; Danger mark 4 (flammable gas), 14 (dangerous).
What are the uses of amine?
Main uses: used in dye synthesis and as an extractant, Emulsifiers, pharmaceutical raw materials, reagents, etc.
I. Health hazards
Invasion: inhalation, ingestion, percutaneous absorption.
Health hazards: Exposure to ethylamine vapors can cause eye irritation, corneal damage, and upper respiratory tract irritation. Liquid splashing into the eyes can cause severe low-level injuries; contaminated skin can cause burns.
Second, emergency treatment and disposal methods
1. Emergency response to leakage
Quickly evacuate personnel from the leaked contaminated area to the upper reaches and isolate them to strictly restrict access. Cut off the fire. It is recommended that emergency handlers wear self-contained positive-pressure respirators and fire protective clothing. Cut off the source of the leak if possible. If it is gas, cover the sewer near the leak with an industrial coating or absorbent/absorbent to prevent gas from entering.
Reasonable ventilation to accelerate diffusion. Spray water is diluted and dissolved. Construct a large amount of wastewater generated by the construction of a dike or pit. If possible, use the exhaust fan to send the brutal residual gas or leaked gas to the washing tower or the fume hood connected to the tower.
The leaking container should be properly handled, reused after repair and inspection. If liquid, absorb with sand, vermiculite or other inert materials. If there is a large amount of leakage, construct a dyke or dig to contain it; cover it with foam to reduce the steam disaster.
Use the explosion-proof pump to transfer to a tanker or special collector, recycle or transport to waste disposal place for disposal. The storage tank area is best equipped with dilute acid spraying facilities.
Waste disposal method: Use controlled incineration. The nitrogen oxides discharged from the incinerator are removed by a scrubber or a high-temperature device.
2.Protective measures
Respiratory protection: When the concentration in the air exceeds the standard, wear a filtering gas mask (half-mask). It is recommended to wear an oxygen respirator or air respirator for emergency situations or rescue.
Eye protection: Wear chemical safety protective glasses.
Body protection: Wear protective clothing.
Hand protection: Wear rubber gloves.
Others: Smoking, eating, and drinking are strictly forbidden at the worksite. After work, take a shower and change clothes.
3.First-aid measures
Skin contact: Remove contaminated clothing immediately and rinse with plenty of running water for at least 15 minutes. Seek medical attention.
Eye contact: Raise the eyelids immediately and rinse thoroughly with plenty of running water or saline for at least 15 minutes. Seek medical attention.
Inhalation: Quickly leave the scene to fresh air. Keep your airways open. If breathing is difficult, give oxygen. If not breathing, give artificial respiration immediately. Seek medical attention.
Ingestion: Rinse mouth with water and drink milk or egg white. Seek medical attention.
Fire fighting method: cut off the air source. If the gas source cannot be cut off immediately, the burning gas is not allowed to be extinguished. Spray water to cool the container. If possible, move the container from the fire to an open area. Fire extinguishing agent: mist water, alcohol-resistant foam, dry powder, carbon dioxide.