Benzene ring properties and structure || What is Benzene Used for?
After the discovery of any organic compound, its properties are studied and its structure is established on the basis of its properties. The structure of benzene and benzene ring is thus established.
Molecular Formula
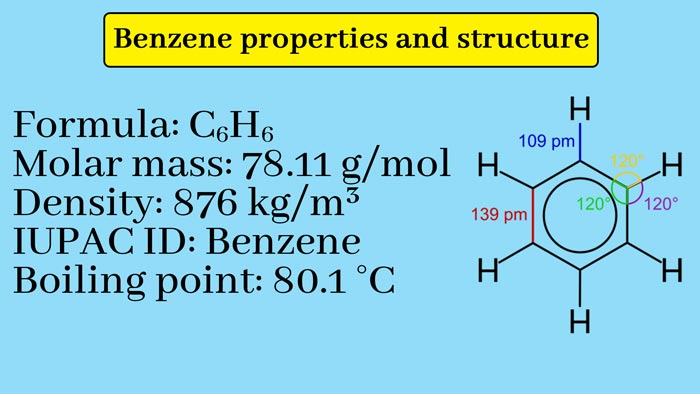
Based on the quantitative determination of elements and the use of molecular weight determination, it is known that the molecule formula of benzene is C6H6.
Open Chain Structure of Benzene
The molecule of the corresponding alkane of benzene is C6H14 according to the formula CnH2n. Benzene ring has eight hydrogen atoms less than it. Therefore, if the carbon atom forms an open chain in the structure of benzene, it should have four double bond or corresponding double bond and three bond. On this basis, the following open chain structures of benzene are possible.
(A) HC=C-CH2-CH2-C=CH
(B) H2C=CH-C≡C-CH=CH2
(C) H3C-C≡C-C≡C-CH3
Open chain structures of benzene are not possible due to the following reasons.
I – The above structures demonstrate that, like ethylene and other aliphatic unsaturated hydrocarbons, benzene will also color Br2/CCl4 and change the color of the Bayer reagent. Benzene does not do this. Hence the above structure of benzene is defective.
II – Benzene exhibits halogensation, nitrification, sulfonization, and other substitution reactions easily. In these reactions one or more hydrogen atoms present in the benzene molecule are displaced by other atoms or groups.
III – Aliphatic unsaturated hydrocarbons do not exhibit this type of reaction. Therefore, these reactions of benzene cannot be explained on the basis of the above structures.
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
- What is Urea || How to make Urea Fertilizer, || Urea uses
The above structures show that one molecule of benzene will add four molecules of hydrogen. In fact, one molecule of benzene is the sum of three molecules of hydrogen. Therefore, the above structures of benzene ring are defective.
From the above discussion, it is clear that the detailed chain structure of benzene is not possible, there are three carbon-carbon two-bound present and the nature of the double-bond present in it is different from the nature of double-bond present in the unsaturated hydrocarbons.
Benzene Ring Structures
Wide chain structures of benzene ring are not possible. Hence its structure is cyclic. There are three twins in it. This gives only one mono-substitution product, meaning that the positions of all six hydrogen atoms in it are the same. Based on these facts, its three cyclical structures are possible.
Benzene ring gives three isomeric double-substitution products. Only 2 – 2 isomeric double-substitution products are possible from structures I and II. Three isotropic two-substitution products are possible from structure III.

On this basis, structures I and II of benzene are not possible and structure III is possible structure of benzene. This structure was proposed by kekule in 1865 and is called Kekule structure.
Kekule’s Dynamic Formula
The two-substituted products of Structure III are as follows:
1, 2-product or ortho-product
1, 3-product or meta-product
1, 4-product or pera-product
A closer study of structure III suggests that two ortho products are possible.

These two products are different from each other. The reason for this is that one of these products does not have a binding between the carbon atoms that carry the substituent group, while the other product has a binding between the carbon atoms that sister the substituent group.
Thus a total of four double-substitution products are possible from the structure of the cake, while a total of three double-substitution products are obtained from benzene.
kekule introduced the kinetic formula of benzene in 1872 to correct this defect of benzene’s structure III. According to the kinetic formula of kekule, benzene ring is an equilibrium mixture of the following two structures, in which these two structures rapidly change into each other.
Since the two Ortho-replacement products rapidly change into each other, they cannot be separated and are considered to be the same product.
Kekule’s Benzene Ring Structure:
The following evidence is prominent in favour of the formula of Kekule
- This explains the sum of three molecules of hydrogen on one molecule of benzene, three molecules of chlorine and three molecules of ozone.
- This explains the formation of one mono-substitution and three double-substitution products from benzene.
- This explains the synthesis of benzene from acetylene.
- This explains the formation of 3 molecules of glycals by ozonisation of benzene.
- Spectral analysis of benzene and its parachor value confirm its formula
The formula of Kekule gives satisfactory explanation of most of the properties of benzene but there are some deficiencies left in it.

Three carbon-carbon bifurcations are present in the Kekule structure of benzene. Despite this, benzene additive reactions do not show up easily. It does not change the color of Br2 / CCl4 and does not change the color of the Bayer reagent. In contrast, benzene substitution reactions are simpler. This behavior of benzene is not clear by the Kekule formula.
Hydrogenation heat data show that the structure of benzene is more stable than that of the mostly unsaturated compounds. The reactions of benzene also show the stability of its structure. Most of the reactions of benzene are such while benzene resists oxidation due to the stability of the cyclic structure. The structure of the capule is unable to explain the durability of the cyclic structure of benzene.
Modern Benzene Ring Structure
According to modern ideas the structure of benzene is clarified based on the following concepts.
Resonance Concept
According to this concept, benzene is considered to be a resonance hybrid of both kekule’s structures.
The actual structures of benzene are neither I nor II but are the mean of both these structures. All its properties cannot be interpreted from Structure I or II but from the mean of Structure I and II.
Therefore, each carbon-carbon bond length in benzene is between 1.54A° between single mid-length 1.34A° and double-bond length 1.39A° The major effect of resonance is that the durability of resonant hybrids is greater than the durability expected from resonant structures. Thus the resonant structure of benzene also explains its stability.
Molecular Orbital Concept
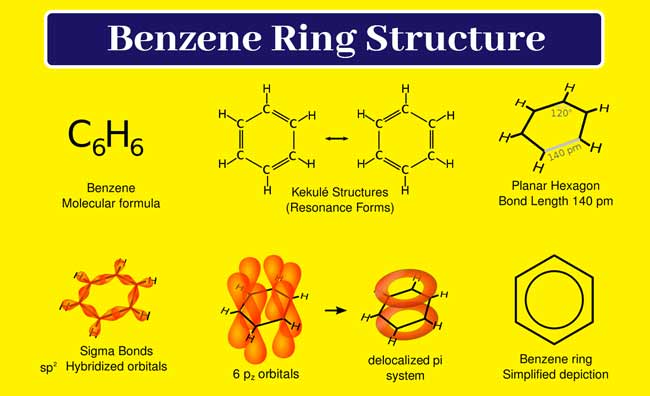
According to this determination, 6 carbon atoms in a benzene molecule are present in a cyclic chain. Each carbon atom is sp2 hybridized. Three sp2 hybridized orbitals of each carbon atom are used to form three sigma bonds. Each carbon atom is made up of one sigma bond with one hydrogen atom and one sigma bond with the adjacent carbon atom.
Thus these 6 carbon atoms form a rhombus in benzene having C – C -H and C – C -C bond angles of 120° and one unused P orbital remaining on each carbon atom. All these p orbitals are parallel to each other.
Each p orbital can overlap with its left or right p orbital to form a pie bond. According to the molecular orbital concept, the pie electrons undergo delocalization and form an unstructured molecular orbital consisting of 6 pie electrons. The stability of this molecule is greater than expected due to delocalization.
- Why are aromatic compounds called aromatic? | Definition, Properties
- What is toluene used for? Preparation and Properties
- Ethyl Amine: Preparation, Properties, Uses, and Tests
- Amines: Nomenclature, Isomerism, Basic Characters
- How is Ethyl Acetate made? | Properties | Uses
- Is Acetamide base or acid? | Preparation | Properties | Uses
- Acetic Anhydride: What is acetic anhydride used for?| Preparation | Properties
- Acetyl chloride: How do you make Acetyl Chloride? | Properties | Uses
On the basis of each of the above concepts, all properties of benzene are clearly explained. It is clear that benzene can be represented by capule structures I or II, since both of these structures are not real structures of benzene, so its actual structure is often represented from structure III.