2) The number of electrons in the outermost orbit of the outermost orbit cannot exceed 8 and the number of electrons in the orbit penultimate orbit cannot exceed 18.
For example, the atomic number of potassium (K) is 19, since there can be no more than 8 electrons in the outermost orbit, so its electronic configuration cannot be 2, 8, 9.
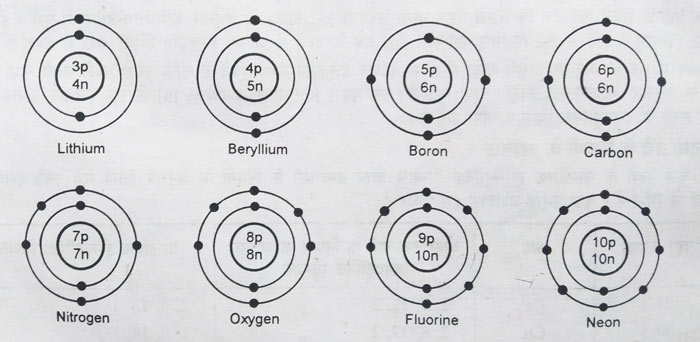
The maximum number of electrons in the first class is 2. After having 2 electrons in the first orbit, electrons start filling up in the second orbit.
- Oxidation Number : How to find Oxidation State
- Bleaching Powder : Preparation, uses of Bleaching Powder
- Iodine : Properties, Preparation and uses
- Bromine – Preparation, Physical and Chemical Properties a Uses
- Chlorine Property : Physical and Chemical Properties | Uses
- Solar Energy : Light Waves, Reactions and Uses
- Chlorine Gas – Laboratory and Industrial Preparation
- Sulfuric Acid : Chemical Properties, Uses and Structure
For example, atomic number of potassium k is 19, after 2 electrons come in its first orbit, 8 electrons come in the second orbit, after that the electrons in the third orbit start filling up.
After the number of electrons in the third orbit is 8, electrons start filling in the fourth orbit. Therefore, the electronic configuration of potassium is 2, 8, 8, 1.
The maximum number of electrons in the first class is 2. After having
2 electrons in the first orbit, electrons start filling up in the
second orbit. After this, after having 8 electrons in any orbit,
electrons start filling in the next orbit.

For example, atomic number of potassium k is 19, after 2 electrons come in its first orbit, 8 electrons come in the second orbit, after that the electrons in the third orbit start filling up. After the number of electrons in the third orbit is 8, electrons start filling in the fourth orbit. Therefore, the electronic configuration of potassium is 2, 8, 8, 1.
The outermost orbit can have more than 2 electrons only when the number of electrons in the penultimate orbit is maximized according to the 2n2 rule. For example, atomic number of titanium (ti) is 22, followed by 2 electrons in its first orbit, followed by 8 electrons in the second orbit.
After this, electrons start filling up in the third orbit. The fourth orbit cannot have more than 2 electrons as the third orbit has 8 electrons and according to the 2n2 law it can have a maximum of 18 electrons.
Hence, the remaining 2 electrons go to the third orbit. Hence the electronic configuration of titanium is 2, 8, 10, 2.
The penultimate orbit can have more than 8 electrons only if the number of electrons in the orbit before it is maximized according to the 2n rule.
For example, the electronic configuration of titanium is 2, 8, 10, 2. Its penultimate orbit has more than 8 electrons since the orbit before it has 8 electrons which is the maximum number of electrons in that orbit according to the 2n2 rule.
Bohr-Bury scheme Facts
The above rules of Bohr and Bury are based on Bohr and Somerfield’s atomic models. After the modern form of nuclear structure was revealed, it has become clear that the rules of Bohr and Bury now have only historical significance.
Modern methods are now used to find the electronic configuration of elements. The modern method is better than Bohr’s acquittal.
With the help of the Bohr Bury scheme only the number of electrons of the main energy levels of the atom are known. According to the Aufbau rule, the number of electrons of the sub-energy levels of an atom is also known.
According to the Aufbau principle, the method of determining the distribution of electrons in atoms is more convenient than the Bohr acquittal scheme.
The electronic configuration of some elements known by the Bohr Bury scheme and the Aufbau principle is different from their actual electronic configurations. The number of these exceptions is not less than the Aufbau principle for the Bohr-Bury scheme.
Electronic configurations of elements can be written with the help of Bohr and Bari rules.
Hydrogen and helium atoms have 1 and 2 electrons in the first orbit, respectively.

There can be a maximum of 2 electrons in the first orbit, so for
elements with atomic numbers 3 and more than 3, the electrons start
entering the second orbit. This sequence until the second orbit has a
maximum of 8 electrons according to the 2n2 law. Thus electronic configurations of elements from Li to Ne are obtained.
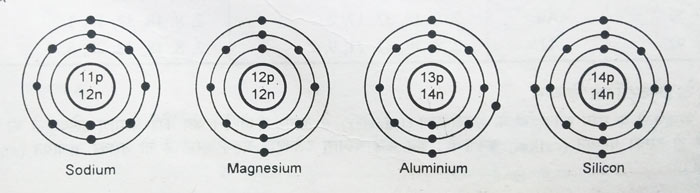
After having 8 electrons in the second orbit, for elements with atomic number 11 and more than 11, the electrons start entering the third orbit. Thus electronic configurations of elements ranging from Na atomic number = 11 to Ar atomic number = 18 are obtained.
The last electron in an atom of K (atomic number = 19) cannot enter the third orbit since according to the above rule, the outermost orbit cannot have more than 8 electrons.
When 8 electrons are complete in any orbit, they start filling electrons in the next orbit.
In this way, the last electron in the atoms of K(atomic = 19) and Ca (atomic number = 20) enters the fourth orbit.
Thus the electronic configurations of K and Ca are obtained. After filling 2, 8, 8, 2 electrons in the orbitals of Sc (atomic number = 21), the last electron cannot enter the fourth orbit because the outermost orbit cannot have more than 2 electrons unless the orbit before it. According to the 2n rule, the maximum electron should not be fulfilled.
Therefore, the final electrons in elements from Sc (atomic number = 21) to Zn (atomic number = 30) gain entry into the third class. More than 8 electrons can orbit in the third orbit since the first orbit has the maximum number of electrons according to the 2n2 law.
Thus electronic configurations of elements ranging from k (atomic number = 19) to Zn (atomic number = 30) are obtained.
Electronic Configuration of Ions
Ions are formed by the transfer of electrons between atoms. When an atom renounces the electron, its cation is formed. When an atom accepts an electron, its anion is formed.
odium atom sodium ion
Na – e⁻ → Na⁺
Atomic Number = 11
Number of electrons = 11
Electronic configuration = 2, 8, 1
Atomic Number = 11
Number of electrons = 10
Electronic configuration = 2, 8
Calcium atom calcium ion
Ca – 2e → Ca⁺⁺
Atomic Number = 20 Atomic Number = 20
Number of electrons = 20 Number of electrons = 18
Electronic configuration = 2, 8, 8, 2 Electronic configuration = 2, 8, 8
Oxygen atom oxide ion
O + 2e⁻ → O⁻⁻
Atomic Number = 8 Atomic Number = 8
Number of electrons = 8 Number of electrons = 10
Electronic configuration = 2, 6 Electronic configuration = 2, 8
Chlorine atom chloride ion
Cl + e⁻ → Cl⁻
Atomic Number = 17 Atomic Number = 17
Number of electrons = 17 Number of electrons = 18
Electronic configuration = 2, 8, 7 Electronic configuration = 2, 8, 8
