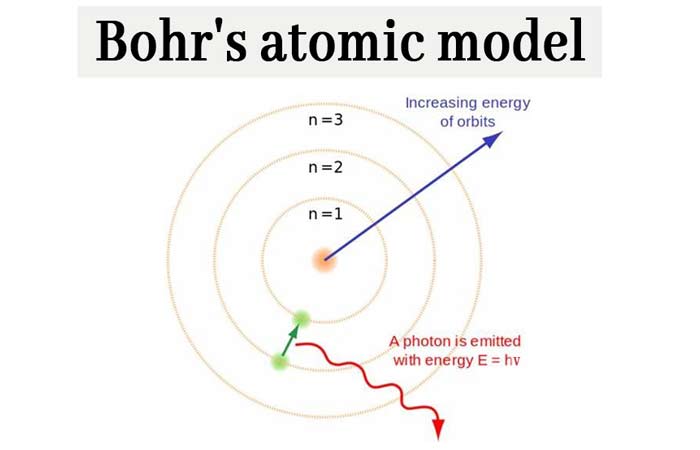
Bohr’s Atomic Model: What is Bohr’s model of an atom? chemistry Notes
Thomson’s Atomic Model
J. J. Thomson presented the first model of the atom in 1904. It is also called Tomson’s Watermelon Model. According to this, the atom is made up of electrons and positive charged matter. The atomic spherical is a very small and electrically neutral particle. we discus about bohrs atomic model in below secession.
Positive charged in the atom is spread evenly. And the electrons are trapped in the charged material in such a way that the watermelon contains the seeds. After Rutherford’s use of a particle scattering, it has been proven that this model of Thomson is faulty.
Rutherford’s Atomic Model
An atom consists of electrons and protons, in an atom, protons are in its nucleus and electrons are outside the nucleus.
1) In an atom, the electrons rotate in circular orbits around the nucleus.
2) The nucleus of the atom attracts the electron of the atom but the centrifugal force generated by the circular motion balances this force.
3) An atom consists of electrons and protons, in an atom, protons are in its nucleus and electrons are outside the nucleus.
4) In an atom, the electrons rotate in circular orbits around the nucleus.
5) The nucleus of the atom attracts the electron of the atom but the centrifugal force generated by the circular motion balances this force.
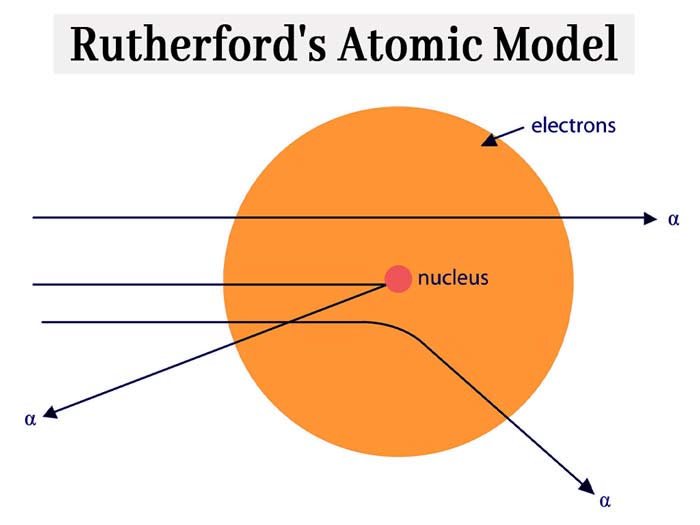
Rutherford’s nuclear model of atomic structure is similar to that of the
solar system, in which various planets always revolve around the Sun.
The following shortcomings have been found in Rutherford’s atomic model – unlimited
circular orbits are possible around the nucleus, in which orbits the
electrons rotate, their velocity and what are their numbers in different
orbits are not known from Rutherford’s model.