Chemical Equilibrium: Characteristics, Types, Examples, Constant
- Calcium carbonate – CaCO3
- Acetic acid – CH₃COOH
- Ethyl acetate – C4H8O2
Understand the chemical equilibrium with an example:
Following is the equation for the reversible reaction of iron and steam.
3Fe + 4H2O = Fe3O4 + 4H2
If steam is passed over the iron in an open tube in its heated state, the iron is almost completely converted into magnetic oxide. In this case, the forward reaction displayed in the above equation achieves almost perfect.
The reason for this is that the hydrogen gas produced as a result of the reaction keeps coming out and the reactants of the opposite reaction are not available simultaneously. Similarly, if hydrogen gas is passed over an open tube in a heated state of iron magnetic oxide, it is almost completely converted into iron.
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
In this case, the opposite reaction shown in the above equation achieves almost perfect. In this case, the water vapor formed as a result of the reaction keeps coming out and the reactants of the front reaction are not available simultaneously.
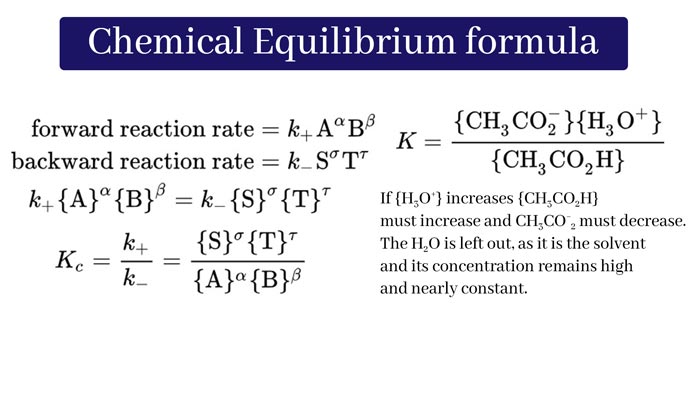
What is meant by chemical equilibrium?
If such reactions are made in a closed container, then initially the velocity of the forward reaction is high and the velocity of the opposite reaction is zero. As the quantities of the reactants decrease and the quantities of the products increase, the velocity of the forward reaction decreases. And the velocity of the opposite reaction increases.
This is how a situation occurs. When the velocities of the front and reverse reactions are equal. This degradation of a reversible reaction is called a chemical Equilibrium.
The state of a reversible reaction in which the quantities of reactants and products are constant and do not change with time is called Chemical Equilibrium.

In the chemical equilibrium, the quantities of the reactants and products remain constant due to the velocity of the front and reverse reactions being equal. It does not change over time. Therefore, the definition of chemical equilibrium can also be given in this way –The state of a reversible reaction in which the quantities of reactants and products are constant and do not change with time is called chemical equilibrium.
Important Characteristics of Chemical Equilibrium
The following are the major features of chemical equilibrium:
In chemical equilibrium, the velocity of frontal and reverse reactions are equal.
Equilibrium can be achieved by initiating the reaction in any direction, forward and reverse.
At equilibrium, there is no change in the observable properties of the reaction mixture such as color and measurable properties such as volume, concentration, volume, and pressure.
In chemical equilibrium, the quantities and concentrations of reactants and products are constant and do not change over time.
Chemical equilibrium is dynamic. When increasing or decreasing the concentrations of the reactants or products, the equilibrium shifts to the left and right.
The equilibrium changes when heat, pressure, volume, and concentration changes.
The presence of a catalyst has similar effects on frontal and reverse reactions. Hence the presence of catalyst does not change the equilibrium.
In chemical equilibrium, the free energy change is zero.
Example of Chemical Equilibrium:
Thermal dissolution of calcium carbonate
Calcium carbonate is heated in a vacuum in a closed container at about 60°C or dissociation. And calcium oxide and carbon dioxide gas are formed. Initially, carbon dioxide is produced continuously.
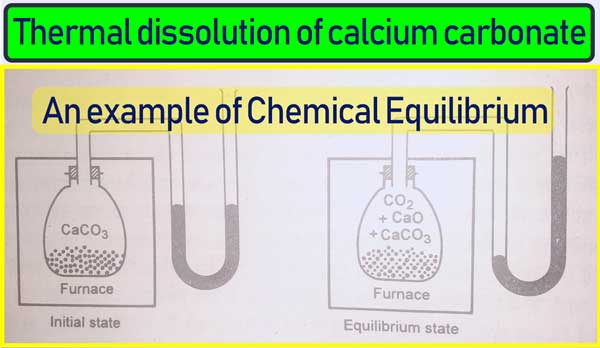
And the pressure displayed by the manometer also keeps increasing. A situation occurs such that the pressure becomes constant. In this case the amount of carbon dioxide does not increase even though there is sufficient calcium carbonate in the vessel. This situation shows the equilibrium of the following reaction.
CaCO3 (s) ⇌ CaO(s) + CO2(g)
The reaction of Hydrogen and Iodine
When a mixture of hydrogen and iodine is heated in a closed vessel at 4440C, the following reaction occurs –
H2(g) + I2(g) ⇌ 2HI (g)
Initially, due to the presence of excess iodine, the color of the mixture is dark purple. Due to the use of iodine in the forward reaction, the color of the mixture gradually becomes lighter. After some time there is no change in the intensity of the color of the mixture. This situation shows the equilibrium of the above reaction.
Types of chemical Equilibrium:
There are two types of chemical Equilibrium
Homogeneous Equilibrium
In this type of equilibrium, all reactants and all products are in the same phase. In homogeneous gaseous equilibrium, all reactants and all products are in a gaseous state.
H2(g) + I2(g) ⇌ 2HI (g)
N2(g) + O2(g) ⇌ 2NO (g)
In homogeneous liquid equilibrium, all reactants and all products are in liquid state.
CH3COOH(l) + C2H5OH(l) ⇌ CH3COOC2H5(l) + H2O(l) *l – liquid
Heterogeneous Equilibrium
In this type of reaction, reactants and products are in two or more phases.
3Fe + 4H2O ⇌ Fe3O4 + 4H2
NEET Previous Year Chemistry Questions
01.
(NEET 2017, 2007)
(a) K2 K33/K1 (b) K2 K3/K1
(c) K32K3/K1 (d) K1 K33 /K2
02 . If the equilibrium constant for (2015)
N2(g) + O2(g) ⇌ 1/2NO(g) is K, the equilibrium constant for
1/2N2(g ) + 1/2O2(g ) ⇌ NO(g ) will be
(a) 1/2K (b) K
(c) K2 (d) K1/2
03. Given that the equilibrium constant for the reaction,
2SO2(g ) ⇌ O2(g ) 2SO3(g )
has a value of 278 at a particular temperature. What is the value of the equilibrium constant for the following reaction at the same temperature?
SO3(g) ⇌ SO2(g) + 1/2O2(g)
(b) 1.8 × 10–3 (b) 3.6 × 10–3
(c) 6.0 × 10–2 (d) 1.3 × 10–5
04. Given the reaction between 2 gases represented by A2 and B2 to give the compound AB(g).
A2(g) + B2(g) ⇌ 2AB(g)
At equilibrium, the concentration of A2 = 3.0 × 10–3 M, of B2 = 4.2 × 10–3 M, of AB = 2.8 × 10–3 M
If the reaction takes place in a sealed vessel at 527°C, then the value of Kc will be
(a) 2.0 (b) 1.9 (c) 0.62 (d) 4.5
05. The pKb of dimethylamine and pKa of acetic acid are 3.27 and 4.77 respectively at T (K). The correct option for the pH of dimethylammonium
acetate solution is:
(a) 8.50 (b) 5.50
(c) 7.75 (d) 6.25
Answer Key
01. (a) 02. (d) 03. (c) 04. (c) 05. (c)