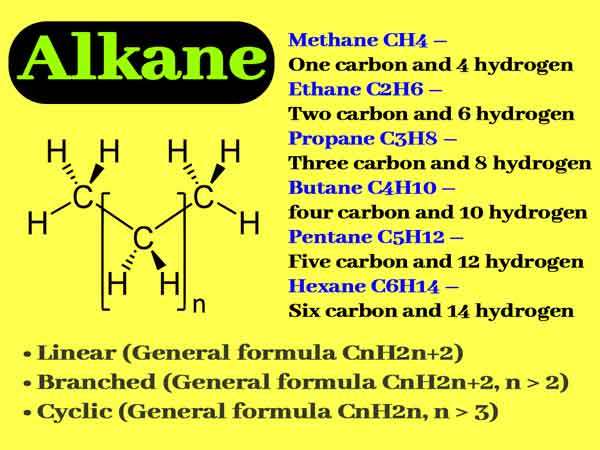
Chemical Properties of Alkanes || What are Examples of Alkanes?
Any of a series of saturated aliphatic hydrocarbons (such as methane, ethane, etc.), such compounds are the main components of petroleum. Alkanes are saturated hydrocarbons, which are chain hydrocarbons with only carbon-carbon single bonds. Chemical properties of alkanes are well defined in NCERT chemistry topics.
They are the simplest type of organic compounds. In alkanes, the number of hydrogen atoms reaches a maximum. Each carbon atom in the molecule is sp3 hybrid. The simplest alkane is methane.
At the same time, the alkane is also a general term for saturated hydrides of many elements.
Categorize as hydride suffix and system
Mononuclear and their names
The “alkane” (-ane suffix) actually refers not only to the saturated hydrides of carbon but can refer to molecular hydrides in which all parent molecules do not contain unsaturated bonds. The mononuclear hydride is directly named after “element + alkane”.
Example:
- AlH3 – Alane
- (CH3CH2CH2)3 B——tri-propyl borane
- (CH3CH2)4 Pb——tetraethyl lead
The use of “Methane” is too common to be mistaken for a system name. In fact, “Carbine” is the systematic name for the substance. Although this name is rarely used, it is good to know its origin.
Some different hydrides of variable elements use the same alkane name, and non-standard bonding number hydrides are prefixed with “λ” to distinguish them. From the table above, we can know the names of generally saturated alkanes.
Standard bonding number: halogen group 1, oxygen group 2, nitrogen group 3, carbon group 4, boron group 3.
Example:
- C6H5-SH5 ——phenyl-λ⁶-sulfane
- (C6H5)3PH2 ——triphenyl-λ⁵-phosphorane
Nomenclature of Alkanes
Beginning with the corresponding number, carbenes do not introduce the carbon element prefix, non-carbohydrates must add the nuclear element prefix.
Example:
- H2N-NH2 ——Azidine
- HS-SH4-SH——2λ⁶-propane sulfane
- PH2(PH)15PH2 -heptadecane
Linear Hexane Nomenclature
When the parent atom is replaced by another atom, the name should be modified by the corresponding prefix of the heteroatom. When the skeleton chain electrons of the alkane are randomly occupied by other heteroatoms, the original name is modified with the heteroatom prefix but the total number of heteroatoms should be included in the total electrons of the skeleton chain.
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
Example:
SiH3 OSiH2O (SiH2)2 OSiH2 SiH3 ——2,4,7-trioxanonylsilane
If the heteroatom and the main chain atom appear alternately on the chain and the elements at the two ends of the chain are the same, the segment atom is named as the heteroatom. Needless to say the number of chain atoms.
Example:
- SiH3 NHSiH3 – disila- disilazane
- NH2 (SiH2 NH)3 SiH2 NH2 —— Pentazocine( C19H27NO )
Alkanes
In an alkane, each carbon atom is tetravalent, and there are only carbon-carbon single bonds and hydrocarbon single bonds. Using sp3 hybrid orbits, it forms a strong σ bond with the surrounding 4 carbon or hydrogen atoms.
The carbon atoms connected to 1, 2, 3, and 4 carbons are called primary, secondary, tertiary, and quaternary carbons respectively; hydrogen atoms on primary, secondary, and tertiary carbons
They are called primary, secondary and tertiary hydrogen.
To minimize the repulsive force of the bond, four atoms connected to the same carbon form a tetrahedron. Methane is a standard regular tetrahedron with a bond angle of 109 ° 28 ′.
In theory, because of the alkane stable structure, all of the alkanes can be stable.
However, alkanes in nature do not exceed 50 carbons at most, and the most abundant alkanes are methane.
Because the carbon atoms in alkanes can be arranged randomly according to the law, the structure of alkanes can be written in countless kinds. Linear alkanes are the most basic structure, and theoretically, this chain can be extended indefinitely. Chemical Properties of Alkanes
It is possible to produce branched chains on the straight chain, which undoubtedly increases the number of alkanes.
Therefore, starting from a 4-carbon alkane, the molecular formula of the same alkane can represent multiple structures. This phenomenon is called isomerism. As the carbon number increases, the number of isomers will increase rapidly.
Alkanes may also undergo optical isomerism. When the four atomic groups connected by a carbon atom are different, this carbon is called chiral carbon, and this substance is optically active.
The remaining part of an alkane that loses a hydrogen atom is called an alkyl group , which is generally represented by R-. Therefore, alkanes can also be represented by the general formula RH.
Alkanes were first named using customary nomenclature. However, this nomenclature is difficult to use for alkanes with many carbon numbers and many isomers. So it was suggested nomenclature derived, all considered as derivatives of methane alkane, for example isobutane, called 2-methylpropane.
Application
Due to the higher cost of alkane production (usually olefins are used for catalytic hydrogenation), alkane is not produced industrially but is directly extracted from petroleum. Since alkane is not easy to react, it is not used as a basic chemical raw material in the industry.
The role of alkanes is mainly to make fuel. Natural gas and biogas (mainly composed of methane) are recently widely used clean energy sources.
Since most of the alkane comes from petroleum, it must be subjected to a fractional distillation process to obtain a variety of alkane for different uses.
Isomer
Different four-carbon hydrocarbons (from left to right): n-butane and isobutane are isomers, and their chemical formulas are both C4H10, cyclobutane and isobutene are isomers, their chemical formulas are C4H8.
Bicyclo [1.1.0] butane is the only alkane with the chemical formula C4H6.
Alkane isomers are mostly chain isomers (isomers due to different branch chains). Alkanes with more than 3 carbon atoms can be arranged in a variety of ways to form isomers. The number of alkane isomers increases as the carbon number increases.
C1: no isomers: methane
C2: no isomers: ethane
C3: two isomers: propane, cyclopropane
C4: two isomers: n-butane, isobutane
C5: three isomers: n-pentane, isopentane, neopentane
C6: five isomers: hexane
C12: 355 isomers
C32: 27711253769 isomers
C60: 22158734535770411074184 isomers, many of which are unstable.
Classification and use
Hexane
Main uses: Extraction solvents for vegetable oils, fish oils, resins, etc., for the manufacture of polyolefin elastomers and pharmaceutical media, and for the production of solvents such as adhesives for tapes, varnishes and tires.
Mixed Heptane
Main use: It can be used as diluent or extender for foreign dry paint adhesive and solvent for organic industry. Multi-Purpose Mineral Spirit is used for paint thinners, ink solvents, glue solvents and general industrial solvents, in addition to alcohol denaturants. Appearance: transparent liquid, no suspended matter
Dry cleaning oil (Stoddard Solvent)
Main use: Excellent dissolving power, it is a dry cleaning solvent for textiles, but it has a slight taste. It has excellent solvent characteristics and is widely used in the manufacture of printing and dyeing, pesticides and herbicides. Appearance: colorless transparent liquid.
Degreasing Oil (Cleaning Naphtha)
Main application: remove oil stains on textiles, washing machine parts, leather degreasing, adhesives and thinners for inks and paints. Appearance: transparent liquid, no suspended matter.
Kerosene (Paraffin oil)
Main use: cleaning machinery, heating and heating, rice drying, tobacco drying and other fuels.
Paint solvents (VM & P Naphtha)
Main uses: solvents, thinners and thinners for paints, enamels, clear lacquers, varnishes, inks, artificial leather, textiles, waterproofing agents, wood preservatives and artificial resins. Appearance: transparent liquid, no suspended matter.
Paint solvent -100, 150, 200 (High-Flash Aromatic Naphtha)
Main application: Solvent with high solubility, widely used for paint preparation such as baking paint, paint, varnish, etc. In industry, it is mostly used as a solvent for x-killer, wood preservative, artificial resin, textile printing and dyeing agent. Appearance: transparent liquid, no suspended matter.
Thinner-170 (Petroleum Thinner-170)
Main application: solvent with high dissolving power. It is mostly used in industry to dilute pesticides to prepare emulsions, wood preservatives, artificial resins, textile printing and dyeing agents, etc. Appearance: transparent liquid, no suspended matter.
Alkanes System Nomenclature
The current nomenclature uses the IUPAC nomenclature. The systematic naming rules for alkanes are as follows:
Find the longest carbon chain as the main chain. Name the main chain according to the number of carbons. The top ten are Tiangan (A, B, C …) representing the carbon number. When the number of carbons is more than ten, name the Chinese numbers, such as Undecane( Formula: C11H24 ).
When there are multiple substituents, use the smallest and longest carbon chain as the main chain, and list all substituents in the order of methyl, ethyl, and propyl.
When more than two substituents are the same, add Chinese numerals in front of the substituents: one, two, three …, such as dimethyl( Formula: C2H6S ), their positions are separated by and listed together in front of the substituents.
Structural formula of isooctane ( 2,2,4-trimethylpentane ). Isooctane is a standard for gasoline knock resistance, with an octane number of 100. For some simple or commonly used alkanes, common names are often used. For example, it is customary to prefix the name of a linear alkane with the word “n”, but the word is not included in the system name.
Those with a methyl group at the 2-position of the main chain are called “iso”, and those with two methyl groups at the 2-position are called “new”. Although this is only suitable for butane and pentane with few isomers, it has been retained due to habit, and even 2,2,4-trimethylpentane, which should not be called “iso”, is also labeled with ” iso ” ” Octane “.
Chemical Properties of Alkanes
Alkanes are very stable because the CH and CC double bonds are relatively stable and difficult to break. Apart from the following three reactions, alkanes can hardly carry out other reactions.
Oxidation Reaction
R + O2 → CO2 + H2O
All alkanes can be burned, and the reaction exothermic. Alkanes complete combustion CO.’S2 and H2O. If the amount of O2 is insufficient, toxic gases such as carbon monoxide (CO) and even carbon black (C) will be generated.
Take methane as an example:
CH4 + 2O2 → CO2 + 2H2O
When the supply of O2 is insufficient, the reaction is as follows:
CH4 + 3/2O2 → CO + 2H2OCH4 + O2 → C + 2H2O
Large-molecular-weight alkanes often cannot be completely burned. When they are burned, black smoke is generated, that is, carbon black. The same is true of the black smoke in the car’s exhaust.
Halogenation reaction
R + X 2 → RX + HX
Because the structure of alkanes is too strong, ordinary organic reactions cannot proceed. Halogenation of alkanes is a free radical substitution reaction, and light energy is required to generate free radicals at the beginning of the reaction.
The following are the steps in which methane is halogenated. This highly exothermic reaction can cause an explosion.
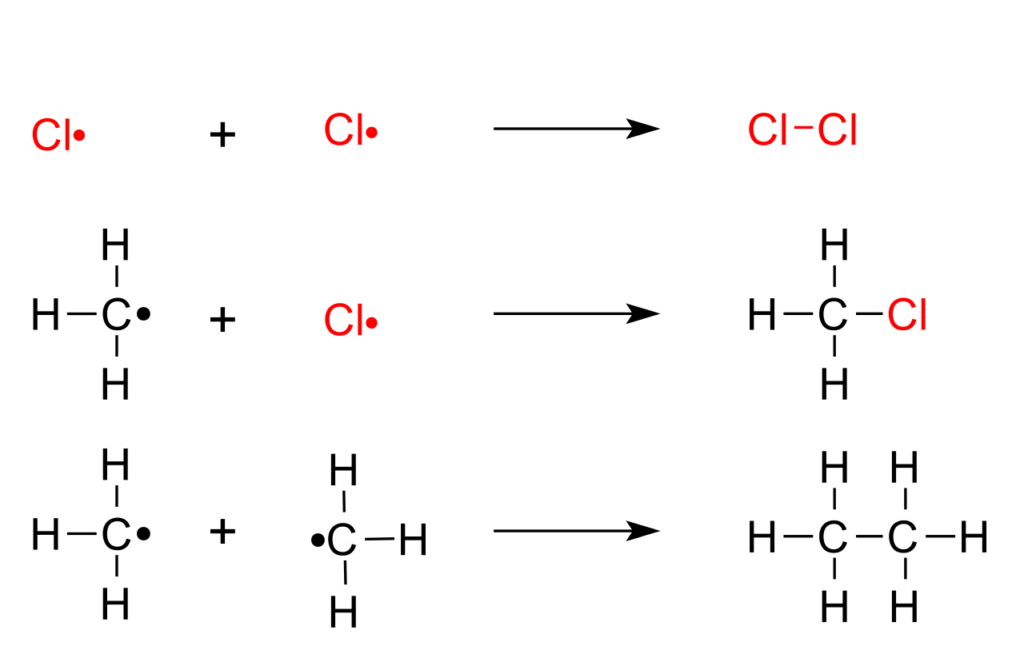
Chain initiation phase: UV a catalyst formed under two Cl the radical
Cl 2 → Cl * / * Cl
Chain growth stage: one H atom is detached from methane; CH 3 Cl begins to form.
CH 4 + Cl * → CH3 + + HCl (slow) CH3 + + Cl2 → CH3Cl + Cl*
Chain termination phase: two free radicals recombination
And Cl* Cl*, or R* and Cl*, or CH3* and CH3*
Cracking Reaction
The cracking reaction is a process in which large molecular hydrocarbons are split into small molecular hydrocarbons under conditions of high temperature, high pressure, or a catalyst. The cracking reaction is an elimination reaction, so the cracking of alkanes always produces olefins.
For example, hexadecane (C16H34) can be cracked to obtain octane and octene (C8H18). The probability of breaking is different due to the different environments of each bond. The following is an example of the cracking of butane:
CH 3 -CH2 -CH2 -CH 3 → CH 4 + CH2 = CH-CH3 during CH3 – CH2 bond breaking, the possibility of 48%.
The CH2 -CH2 bond is broken during the process of CH3 -CH2 -CH2 -CH3 → CH3 -CH3 + CH2 = CH2 with a probability of 38%.
The CH bond is broken during CH3 -CH2 -CH2 -CH3 → CH2 = CH-CH2 -CH3 + H2 with a probability of 14%.
In the cracking reaction, different conditions can trigger different mechanisms, but the reaction process is similar. Carbon radicals are generated during the thermal decomposition process, and carbon positive ions and hydrogen negative ions are generated during the catalytic cracking process. These extremely unstable intermediates undergo steps such as rearrangement, bond cleavage, and hydrogen transfer to form stable small molecule hydrocarbons.
In industry, deep cracking is called cracking, and the products of cracking are all gases, called cracking gas.
The role of alkanes is mainly to make fuel. Natural gas and biogas (mainly composed of methane) are widely used clean energy sources. Various fractions from petroleum fractionation are suitable for various engines:
- C1 ~ C4 (distillates below 40 ° C) are petroleum gas and can be used as fuel.
- C5 ~ C11 (distillate at 40 ~ 200 ℃) is gasoline, which can be used as fuel or chemical raw material.
- C9 ~ C18 (distillate at 150 ~ 250 ℃) is kerosene and can be used as fuel.
- C14 ~ C20 (distillate at 200 ~ 350 ℃) is diesel and can be used as fuel.
Fractions above C20 are heavy oils, and then reduced pressure distillation can obtain substances such as lubricating oil and asphalt.
In addition, the reaction of paraffin to crack olefins has become an important method for the production of ethylene.
Physical Properties
At normal temperature and pressure (1 atmosphere), (methane to butane) is gaseous, between 5 -17 (pentane to heptane) is liquid, and more than 18 carbons (octadecane) are solid.
Alkanes English name
- Methane
- Ethane
- Propane
- Butane
- Pentane
- Hexane
- Heptane
- Octane
- Nonane
- Decane
- Undecane
- Dodecane
- Tridecane
- Tetradecane tetradecane
- Pentadecane
- Hexadecane
- Heptadecane
- Octadecane
- Nonadecane
- Eicosane icosane
