Chlorine Gas Test || How Does Chlorine React with Water
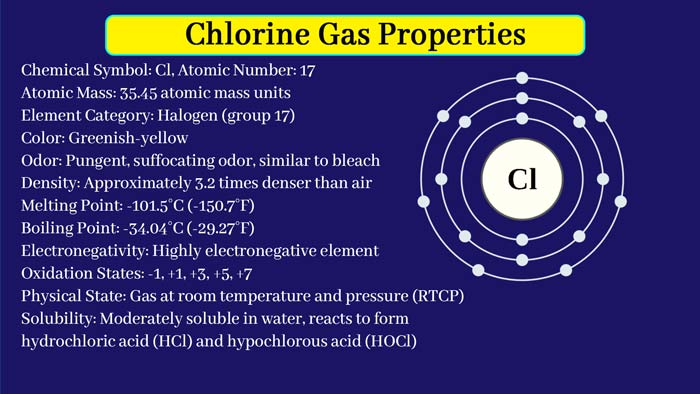
Chlorine is a non-metallic element and belongs to one of the halogens. Chlorine gas is yellow-green gas at normal temperature and pressure, and its chemical properties are very lively and toxic.
It is widely distributed in nature in the form of chemical forms and is also important for the physiological activities of the human body.
Chlorine gas is a yellow-green gas with a density greater than air (3.214 g/L), a melting point of −101.0 °C, and a boiling point of −34.4 °C. It has a strong pungent odor.
Discover a brief history
In 1774, Swedish chemist Scheler engaged Pyrolusite found the study: Pyrolusite mixed with hydrochloric acid heating will produce a suffocating yellow-green gas.
At that time, the great chemist Lavoisier believed that oxygen is the origin of acidity, and all acids contain oxygen.
Scheler and many chemists are convinced that Lavaisier’s view is that this yellow-green gas is a compound composed of oxygen and another unknown group, so Scheler calls it “oxidized hydrochloric acid”.
But the British chemist David holds a different view. He tried every means and could not take the oxygen out of the oxidized hydrochloric acid.
He suspects that there is no oxygen at all in the oxidized hydrochloric acid. In 1810, David proved by irrefutable fact that the so-called oxidized hydrochloric acid is not a compound, but a simple element of a chemical element. He named this element “Chlorine.” Its Greek original meaning is “green.”
Natural Distribution
The free state of chlorine gas in nature exists in the atmosphere and is one of the main elements that destroy the ozone layer. Chlorine is decomposed by ultraviolet light into two chlorine atoms (free radicals). Most are usually in the form of chloride (Cl-), the most common being sodium chloride (salt, NaCl).

Elemental: Cl2
The chlorine element is composed of two chlorine atoms and has a chemical formula of Cl2. The gaseous chlorine single substance is commonly called chlorine gas, and the liquid chlorine single substance is commonly called liquid chlorine.
Chlorine Gas Compound
Inorganic (valence in parentheses): chloride (-1), Hypochlorous acid (+1), hypochlorite (+1), chlorite (+3), chlorite (+3), chlorine Acid (+5), chlorate (+5), Perchloric acid (+7), perchlorate (+7)
Organochlorine Compounds.
Isotope
Chlorine has two stable isotopes, 35Cl and 37Cl. The extranuclear electron configuration is 3S 23P 5. The relative atomic masses are 34.968 852 and 36.965 903, respectively.
The natural abundances were 75.77% and 24.23%, respectively.
Chloride Gas ion Test
To check whether the water contains chloride ions, it can be added with silver nitrated silver ions (such as silver nitrate) (adding acidic silver nitrate can eliminate other ions interference), silver ions and chloride ions will react to form a silver chloride white precipitate, the reaction formula:
Ag ++Cl -→ AgCl
Handling and storage
1) Operation Precautions
Strictly closed, providing adequate local exhaust and full ventilation. Operators must be specially trained to strictly follow the operating procedures.
Operators are advised to wear an air respirator, wear a mask-type tape anti-drug, and wear rubber gloves. Keep away from fire, heat, and smoking in the workplace.
Keep away from flammable and combustible materials. Prevent gas from leaking into the workplace air. Avoid contact with alcohol.
Lightly load and unload during handling to prevent damage to cylinders and accessories. Equipped with the corresponding variety and quantity of fire-fighting equipment and leakage emergency treatment equipment.
2) Storage Precautions
Store in a cool, ventilated warehouse. Keep away from fire and heat. The temperature of the reservoir does not exceed 30°C, and the relative humidity does not exceed 80%. It should be stored separately from combustibles (combustibles), alcohols, and food chemicals.
Avoid mixing. The storage area should be equipped with leakage emergency treatment equipment. The “five pairs” management system for extremely toxic substances should be strictly implemented.
Physical Properties
Chlorine is a yellow-green gas with a density greater than air (3.214 g/L), a melting point of −101.0°C, and a boiling point of −34.4°C. It has a strong pungent odor.
The chlorine gas molecule is composed of two chlorine atoms, slightly soluble in water, easily soluble in alkali liquor, and easily soluble in organic solvents such as carbon tetrachloride and carbon disulfide.
Chlorine has 26 isotopes, of which only 35Cl and 37Cl are stable, and the remaining isotopes are radioactive.
Atomic radius: 100 pm
Extranuclear electronic arrangement: [Ne]3s 23p 5
Valence price: ±1, 3, 5, 7
Crystal structure: orthorhombic system
Electronegativity: 3.16 (Pauling scale)
First ionization energy: 1251.2 kJ/mol
Chemical properties
The outermost electron layer of the chlorine atom has 7 electrons, and it is easy to combine one electron in the chemical reaction so that the outermost electron layer reaches a stable state of 8 electrons.
Therefore, chlorine gas has a strong oxidizing property and can occur with most metals and nonmetals.
The chlorine gas disproportionates to hydrochloric acid and hypochlorous acid, and the hypochlorous acid is unstable and easily liberates free oxygen. Among them, hypochlorous acid has bleaching property (stronger than SO 2 and heating does not restore the primary color).
Chlorine can also be added or substituted with many organic substances and is widely used in life.
Chlorine is highly toxic and has been used as a military gas.
Cl-test
The presence of chloride ions in the water can be added to the soluble silver ions (silver nitrate) (addition of acidic silver nitrate can eliminate other ions interference), silver ions and chloride ions will react to form a silver chloride white precipitate. Then take a white precipitate, add dilute nitric acid, and the precipitate does not dissolve, indicating chlorine ions.
Oxyacid
(1) Preparation
1 chlorine gas in ice water: Cl2 + H2O = HClO + HCl
2 Chlorine in the lye can be obtained hypochlorite: Cl2 + 2NaOH → NaClO + NaCl + H2O
3In the industry, use electrolytic cold brine to stir vigorously to prepare NaClO
(2) Nature
1 is a weak acid, but it is a strong oxidant and has bleaching properties.
2 by oxidation, redox reaction occurs 3ClO- → ClO3 -+ 2Cl –
(3) Use
Production of bleaching powder Ca(ClO)2
Bleaching powder: Cl2 reacts with Ca(OH)2 2Cl2 + 2Ca(OH)2 = Ca(ClO)2+ +CaCl2 + 2H2O
Chlorochloric acid is the only halous acid and is very unstable.
(1) Preparation
1ClO2 decomposes in water:
2ClO2 + H2O = HClO2 + HClO2
2-pass ClO2 in Na2O2 or NaOH and H2O2 can obtain chlorite
2ClO2 + Na2O2 = 2NaClO2 + O2 2ClO2 + H2O2 + OH -= 2ClO2 - +O2 + H2O
(2) Nature and use
1 A very unstable compound, but chlorite is more stable.
2 has bleaching properties
Unstable above 40%
(1) Preparation
① Views chlorate root aqueous solution is heated to produce their oxidation-reduction reaction (disproportionation reaction):
2 Electrolyze the hot sodium chloride aqueous solution and stir it:
3Cl2 + 6OH -→ ClO3 -+ 5Cl -+ 3H2O
(2) Nature and use
Both chloric acid and chlorate are strong oxidants
2 potassium chlorate used to make explosives
3KClO3 is heated by heat
- No catalyst, slightly hot: 4KClO3 = 3KClO4 + KCl (about 100°C)
- Catalyst (MnO2): 2KClO3 = 2KCl + 3O2 (about 300°C)
- Perchloric acid (HClO4) and its salts
1 Low-pressure distillation mixture of KClO4 and H2SO4:
KClO4 + H2SO4 = HClO4 + KHSO4
2 When the brine is electrolyzed, the chlorine produced by the anode is oxidized:
1/2Cl2 + 4H2O = ClO4 - + 8H+ + 7e–
3 chlorate is thermally decomposed:
4KClO3 = 3KClO4 + KCl
(2) Nature and use
1 chlorine is the most stable oxo acid, not easy to decompose
2 very strong acid (the strongest acid in the high school range, stronger than 100% sulfuric acid, but weaker than super acid such as fluoroantimonic acid.
The main purpose Industry
Chlorine is mainly used in the chemical industry, especially in the organic synthesis industry, to produce plastics, synthetic rubbers, dyes, and other chemicals or intermediates, as well as bleaches, disinfectants, synthetic drugs, and the like.
Chlorine is also used in the manufacture of bleaching powders, bleached pulp, and cloth, synthetic hydrochloric acid, chloride production, drinking water disinfection, synthetic plastics, and pesticides.
A lot of chlorine is also needed to extract rare metals.
Physiological
Chlorine is one of the essential macroelements in the body and is essential for maintaining the balance of body fluids and electrolytes.
It is also an essential component of gastric juice. It is often found in the form of chlorides in nature, the most common form being table salt.
Chlorine has an average body content of 1.17g/kg, a total amount of about 82–100g, accounting for 0.15% of body weight, and is widely distributed throughout the body.
The main monochloride form is combined with sodium and potassium. Among them, potassium chloride is mainly in the intracellular fluid, and sodium chloride is mainly in the extracellular fluid.
Dietary chlorine is almost exclusively derived from sodium chloride, and only a small amount is derived from potassium chloride. Therefore, salt and processed food soy sauce, cured meat or smoked food, pickles and salty foods are rich in chloride. In general, the content of chlorine in natural foods varies greatly; almost all natural water contains chlorine.
Main Physiological Functions:
- Maintain body fluid acid-base balance.
- Chloride ion and sodium ion are the main ions that maintain osmotic pressure in the extracellular fluid. The two accounts for about 80% of the total ion number, regulating and controlling the volume and osmotic pressure of the extracellular fluid.
- Participate in blood CO divalent ion transport.
- Chloride ion is also involved in the formation of gastric acid in gastric juice, gastric acid promotes the absorption of vitamin B12 and iron; activates salivary amylase to decompose starch, promotes food digestion, stimulates liver function and promotes the discharge of metabolic waste in the liver, chlorine also stabilizes the membrane potential of nerve cells.
Chlorine gas has a stimulating effect on the eye and respiratory mucosa, which can cause respiratory symptoms such as tearing, coughing, coughing, chest tightness, bronchitis and bronchitis, pulmonary edema, and severe shock and death.
Used as a chemical weapon (suffocating poison) during World War I
Chlorine gas is a serious hazard to the environment and can cause pollution to water bodies.
At the same time, chlorine gas can help combustion, and the wet chlorine gas is highly corrosive.
Therefore, when contacting chlorine gas, it is necessary to pay attention to the strict protection of the whole body. It is strictly forbidden to directly smell and contact with chlorine gas, and the chlorine-containing exhaust gas should not be directly discharged into the atmosphere.